 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| |
| |
| Properties | |
| Te3Cl2 | |
| Molar mass | 453.71 g/mol |
| Appearance | Gray solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
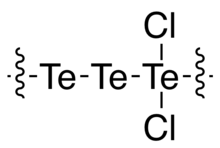
Tritellurium dichloride is the inorganic compound with the formula Te3Cl2. It is one of the more stable lower chlorides of tellurium.
Preparation and properties
Te3Cl2 is a gray solid. Its structure consists of a long chain of Te atoms, with every third Te center carrying two chloride ligands for the repeat unit -Te-Te-TeCl2-.[2] It is a semiconductor with a band gap of 1.52 eV, which is larger than that for elemental Te (0.34 eV).[3] It is prepared by heating Te with the appropriate stoichiometry of chlorine.[4]
Other lower tellurium chlorides
Te2Cl2 (ditellurium dichloride) is a yellow liquid prepared by reaction of lithium polytellurides with TeCl4. Te2Cl, also a polymer, is a metastable gray solid, tending to convert to Te3Cl2 and TeCl4.[3]
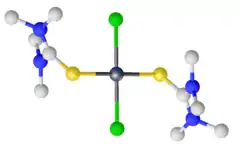
Tellurium dichloride (TeCl2) is unstable with respect to disproportionation, and has not been isolated as a solid, but has been characterised as the main component of the vapor formed with TeCl4 and hot Te.[5] Several complexes of it are known and well characterized. They are prepared by treating tellurium dioxide with hydrochloric acid in the presence of thioureas. The thiourea serves both as a ligand and as a reductant, converting Te(IV) to Te(II).
References
- ↑ Foss, O.; Maartmann-Moe, K. (1986). "Crystal and Molecular Structures of trans Square–Planar Complexes of Tellurium Dichloride, Dibromide and Diiodide with Tetramethylthiourea, TeL2X2. Bond Lengths in Centrosymmetric Tellurium(II) Complexes". Acta Chemica Scandinavica A. 40: 675. doi:10.3891/acta.chem.scand.40a-0675.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- 1 2 Xu, Zhengtao (2006). "Recent Developments in Binary Halogen–Chalcogen Compounds, Polyanions and Polycations". In Devillanova, Francesco (ed.). Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium. Royal Society of Chemistry. pp. 381–416. doi:10.1039/9781847557575-00455.
- ↑ Kniep, R.; Mootz, D.; Rabenau, A. (1976). "Zur Kenntnis der Subhalogenide des Tellurs". Zeitschrift für anorganische und allgemeine Chemie. 422: 17–38. doi:10.1002/zaac.19764220103.
- ↑ Fernholt, Liv; Haaland, Arne; Volden, Hans V.; Kniep, Rüdiger (1985). "The molecular structure of tellurium dichloride, TeCl2, determined by gas electron diffraction". Journal of Molecular Structure. 128: 29–31. doi:10.1016/0022-2860(85)85037-7.