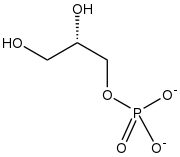
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2S)-2,3-Dihydroxypropyl dihydrogen phosphate | |
| Other names
(S)-2,3-dihydroxypropyl dihydrogen phosphate 1,2,3-propanetriol, 1-(dihydrogen phosphate), (2S)- L-glycerol 1-phosphate D-glycerol 3-phosphate D-α-glycerophosphate D-α-phosphoglycerol glycero-1-phosphate O-phosphonoglycerol 1-phosphoglycerol[1] L-glycerol 1-phosphate D-glycerol 3-phosphate D-α-glycerophosphoric acid[1] | |
| Identifiers | |
3D model (JSmol) |
|
| MeSH | Alpha-glycerophosphoric+acid |
PubChem CID |
|
| UNII | |
| |
| Properties | |
| C3H7O6P | |
| Molar mass | 170.057 g·mol−1 |
| Appearance | colorless |
| Related compounds | |
Related organophosphates |
Glycerol 2-phosphate Glycerol 3-phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
sn-Glycerol 1-phosphate[lower-alpha 1] is the conjugate base of a phosphoric ester of glycerol. It is a component of ether lipids, which are common for archaea.[2]
Biosynthesis and metabolism
Glycerol 1-phosphate is synthesized by reducing dihydroxyacetone phosphate (DHAP), a glycolysis intermediate, with sn-glycerol-1-phosphate dehydrogenase.[3] DHAP and thus glycerol 1-phosphate is also possible to be synthesized from amino acids and citric acid cycle intermediates via gluconeogenesis pathway.
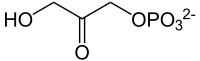 + NAD(P)H + H+ →
+ NAD(P)H + H+ → 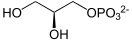 + NAD(P)+
+ NAD(P)+
Glycerol 1-phosphate is a starting material for de novo synthesis of ether lipids, such as those derived from archaeol and caldarchaeol. It is first geranylgeranylated on its sn-3 position by a cytosolic enzyme, phosphoglycerol geranylgeranyltransferase. A second geranylgeranyl group is then added on the sn-2 position making unsaturated archaetidic acid.[4]
Lipid divide
Organisms other than archaea, i.e. bacteria and eukaryotes, use the enantiomer, glycerol 3-phosphate for producing their cell membranes. The fact that archaea use the flipped chirality compared to these two groups is termed a lipid divide.[2] As of 2021, biologists still do not know how the lipid divide happened.[5]
See also
Notes
- ↑ This article uses stereospecific numbering where stereoconfiguration is not explicitly specified.
- 1 2 G. P. Moss (ed.). "Nomenclature of Phosphorus-Containing Compounds of Biochemical Importance". Archived from the original on 2016-12-08. Retrieved 2015-05-20.
- 1 2 Caforio, Antonella; Driessen, Arnold J.M. (2017). "Archaeal phospholipids: Structural properties and biosynthesis" (PDF). Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1862 (11): 1325–1339. doi:10.1016/j.bbalip.2016.12.006. PMID 28007654. S2CID 27154462.
- ↑ Nishihara & Koga (1995). "sn-Glycerol-1-phosphate dehydrogenase in Methanobacterium thermoautotrophicum: key enzyme in biosynthesis of the enantiomeric glycerophosphate backbone of ether phospholipids of archaebacteria". J. Biochem. 117 (5): 933–935. doi:10.1093/oxfordjournals.jbchem.a124822. PMID 8586635.
- ↑ Koga & Morii (2007). "Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations". Microbiol. Mol. Biol. Rev. 71 (1): 97–120. doi:10.1128/mmbr.00033-06. PMC 1847378. PMID 17347520.
- ↑ Sohlenkamp, C (July 2021). "Crossing the lipid divide". The Journal of Biological Chemistry. 297 (1): 100859. doi:10.1016/j.jbc.2021.100859. PMC 8220414. PMID 34097872.