RIG-I (retinoic acid-inducible gene I) is a cytosolic pattern recognition receptor (PRR) that can mediate induction of a type-I interferon (IFN1) response.[5] RIG-I is an essential molecule in the innate immune system for recognizing cells that have been infected with a virus. These viruses can include West Nile virus, Japanese Encephalitis virus, influenza A, Sendai virus, flavivirus, and coronaviruses.[5][6]
RIG-I is an ATP-dependent DExD/H box RNA helicase that is activated by immunostimulatory RNAs from viruses as well as RNAs of other origins. RIG-I recognizes short double-stranded RNA (dsRNA) in the cytosol with a blunt 5' end, 5' tri- or di-phosphate end or a 5' 7-methyl guanosine (m7G) cap (cap-0), but not RNA with a 5' m7G cap having a ribose 2′-O-methyl modification (cap-1).[7][8] These are often generated during a viral infection but can also be host-derived.[5][9][10][11][12] Once activated by the dsRNA, the N-terminus caspase activation and recruitment domains (CARDs) migrate and bind with CARDs attached to mitochondrial antiviral signaling protein (MAVS) to activate the signaling pathway for IFN1.[5][9]
Type-I IFNs have three main functions: to limit the virus from spreading to nearby cells, promote an innate immune response, including inflammatory responses, and help activate the adaptive immune system.[13] Other studies have shown that in different microenvironments, such as in cancerous cells, RIG-I has more functions other than viral recognition.[10] RIG-I orthologs are found in mammals, geese, ducks, some fish, and some reptiles.[9] RIG-I is in most cells, including various innate immune system cells, and is usually in an inactive state.[5][9] Knockout mice that have been designed to have a deleted or non-functioning RIG-I gene are not healthy and typically die embryonically. If they survive, the mice have serious developmental dysfunction.[9] The stimulator of interferon genes STING antagonizes RIG-I by binding its N-terminus, probably as to avoid overactivation of RIG-I signaling and the associated autoimmunity.[14]
Structure
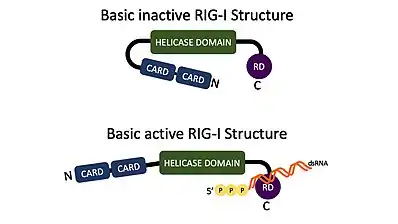
RIG-I is encoded by the DDX58 gene in humans.[9][15] RIG-I is a helical ATP-dependent DExD/H box RNA helicase with a repressor domain (RD) on the C-terminus that binds to the target RNA.[5][9] Included on the N-terminus are two caspase activation and recruitment domains (CARDs) that are important for interactions with mitochondrial antiviral signaling protein (MAVS).[5][9] RIG-I is a member of the RIG-I like receptors (RLRs) that also includes Melanoma Differentiation-Associated protein 5 (MDA5) and Laboratory of genetics physiology 2 (LGP2).[5][9] RIG-I and MDA5 are both involved in activating MAVS and triggering an antiviral response.[16]
Functions
As a Pattern Recognition Receptor
Pattern Recognition Receptors
Pattern Recognition Receptors (PRRs) are a part of the innate immune system used for recognizing invaders.[17] In a viral infection, a virus enters a cell, and it takes over the cell's machinery to self replicate. Once a virus has begun replication, the infected cell is no longer useful and potentially harmful to its host, and the host's immune system must be notified. RIG-I functions as a pattern recognition receptor and PRR's are the molecules that start the notification process. PRRs recognize specific Pathogen-Associated Molecular Patterns (PAMP).[17] Once the PAMP is recognized, it can then lead to a signaling cascade producing an inflammatory response or an interferon response. PRRs are located in many different cell types, however most notably active in the innate immune system cells. In addition, they are located in many different parts of those cells, such as the cell membrane, endosomal membrane, and in the cytosol, to provide the most protection against many types of invaders (i.e., extracellular and intracellular microbes).[5]
RIG-I PAMPs
RIG-I is located in the cytoplasm where its function is to recognize its PAMP, which are ideally short (<300 base pairs) dsRNA with a 5′ triphosphate (5′ ppp).[5][9] However, it has been noted that while not ideal, and response is weakened, RIG-I can recognize 5′ diphosphate (5′pp). This ability is important as many viruses have evolved to evade RIG-I, so having the dual ligand opens up more doors for recognition.[5][9] An example of viruses evolving to evade RIG-I is in the case of certain retroviruses, such as HIV-1, encode a protease that directs RIG-I to the lysosome for degradation, and thereby evade RIG-I mediated signaling.[6] The dsRNA can come from single-stranded RNA (ssRNA) viruses or from dsRNA viruses. The ssRNA viruses are not typically recognized as ssRNA, but through intermittent replication products in the form of dsRNA.[5][9] RIG-I is also able to detect non-self 5′-triphosphorylated dsRNA transcribed from AT-rich dsDNA by DNA-dependent RNA polymerase III (Pol III).[18] It is important to note, however, that the ligands of RIG-I are still being investigated and are controversial. Also notable, is that RIG-I can work together with MDA5 against viruses that RIG-I itself may not create a significant enough response.[5][9] In addition, for many viruses, effective RIG-I-mediated antiviral responses are dependent on functionally active LGP2.[18] Cells are synthesizing multiple types of RNA at all times, so it is important that RIG-I is not going to bind to those RNAs. Native RNA from inside the cell contains an N1 2'O-Methyl self RNA marker that deters RIG-I from binding.[9][10]
Type-1 Interferon Pathway
RIG-I is a signaling molecule and is usually in a condensed resting state until it is activated. Once RIG-I is bound to its PAMP, molecules such as PACT and zinc antiviral protein short isoform (ZAPs), help keep RIG-I in an activated state which then keeps the caspase activation and recruitment domains (CARDs) ready for binding.[5] The molecule will migrate to the mitochondrial antiviral signaling protein (MAVS) CARD domain and bind.[5][9] RIG-I CARD interactions have their own regulatory system. Although RIG-I expresses a CARD at all times, it must be activated by the ligand before it will allow both CARDs to interact with the MAVS CARD.[9] This interaction will start the pathway to making proinflammatory cytokines and type-1 Interferon (IFN1;IFNα and IFNβ), which create an antiviral environment.[5][9] Once the IFN1s leave the cell, they can bind to IFN1 receptors on the cell surface from which they came from, or other cells close by.[9] This will upregulate the production of more IFN1s, boosting an antiviral environment.[5][9] IFN1 also activates the JAK-STAT pathway, leading to the production of IFN-stimulated genes (ISGs).[13]
In Cancer Cells
Usually, RIG-I recognizes foreign RNA. However, it can sometimes recognize "self" RNAs. RIG-I has been shown to enable breast cancer cells (BrCa) to resist treatments and grow because of an IFN response to noncoding RNA. In contrast, RIG-I in other types of cancer, such as acute myeloid leukemia and hepatocellular carcinoma, can act as a tumor suppressor.[10] If cancer causing viruses infect a cell, however, RIG-I can lead to cell death. Cell death can occur via apoptosis via the caspase-3 pathway, or through IFN-dependent T cells and natural killer cells.[19]
Identification and naming
RIG-I was named by researchers from the Shanghai Institute of Hematology who identified novel genes that respond to all-trans retinoic acid (ATRA) in leukemia cells.[20] RIG-I and the other genes were assigned the temporary name of RIG (retinoic acid–induced gene) in the format of RIG-A, RIG-B etc by the group, however they performed no additional characterization on RIG-I.
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000107201 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000040296 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Kell AM, Gale M (May 2015). "RIG-I in RNA virus recognition". Virology. 479–480: 110–121. doi:10.1016/j.virol.2015.02.017. PMC 4424084. PMID 25749629.
- 1 2 Solis M, Nakhaei P, Jalalirad M, Lacoste J, Douville R, Arguello M, et al. (February 2011). "RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I". Journal of Virology. 85 (3): 1224–1236. doi:10.1128/JVI.01635-10. PMC 3020501. PMID 21084468.
- ↑ Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M, et al. (October 2014). "Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5'-diphosphates". Nature. 514 (7522): 372–375. Bibcode:2014Natur.514..372G. doi:10.1038/nature13590. PMC 4201573. PMID 25119032.
- ↑ Devarkar SC, Wang C, Miller MT, Ramanathan A, Jiang F, Khan AG, et al. (January 2016). "Structural basis for m7G recognition and 2'-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I". Proceedings of the National Academy of Sciences of the United States of America. 113 (3): 596–601. Bibcode:2016PNAS..113..596D. doi:10.1073/pnas.1515152113. PMC 4725518. PMID 26733676.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Brisse M, Ly H (2019). "Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5". Frontiers in Immunology. 10: 1586. doi:10.3389/fimmu.2019.01586. PMC 6652118. PMID 31379819.
- 1 2 3 4 Xu XX, Wan H, Nie L, Shao T, Xiang LX, Shao JZ (March 2018). "RIG-I: a multifunctional protein beyond a pattern recognition receptor". Protein & Cell. 9 (3): 246–253. doi:10.1007/s13238-017-0431-5. PMC 5829270. PMID 28593618.
- ↑ Chiang JJ, Sparrer KM, van Gent M, Lässig C, Huang T, Osterrieder N, et al. (January 2018). "Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity". Nature Immunology. 19 (1): 53–62. doi:10.1038/s41590-017-0005-y. PMC 5815369. PMID 29180807.
- ↑ Zhao Y, Ye X, Dunker W, Song Y, Karijolich J (November 2018). "RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection". Nature Communications. 9 (1): 4841. Bibcode:2018NatCo...9.4841Z. doi:10.1038/s41467-018-07314-7. PMC 6242832. PMID 30451863.
- 1 2 Ivashkiv LB, Donlin LT (January 2014). "Regulation of type I interferon responses". Nature Reviews. Immunology. 14 (1): 36–49. doi:10.1038/nri3581. PMC 4084561. PMID 24362405.
- ↑ Yu P, Miao Z, Li Y, Bansal R, Peppelenbosch MP, Pan Q (January 2021). "cGAS-STING effectively restricts murine norovirus infection but antagonizes the antiviral action of N-terminus of RIG-I in mouse macrophages". Gut Microbes. 13 (1): 1959839. doi:10.1080/19490976.2021.1959839. PMC 8344765. PMID 34347572.
- ↑ "DDX58 DExD/H-box helicase 58 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-02-29.
- ↑ Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ (August 2011). "MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response". Cell. 146 (3): 448–461. doi:10.1016/j.cell.2011.06.041. PMC 3179916. PMID 21782231.
- 1 2 Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR (2018). "Pattern Recognition Receptors and the Host Cell Death Molecular Machinery". Frontiers in Immunology. 9: 2379. doi:10.3389/fimmu.2018.02379. PMC 6232773. PMID 30459758.
- 1 2 Satoh T, Kato H, Kumagai Y, Yoneyama M, Sato S, Matsushita K, et al. (January 2010). "LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses". Proceedings of the National Academy of Sciences of the United States of America. 107 (4): 1512–1517. Bibcode:2010PNAS..107.1512S. doi:10.1073/pnas.0912986107. PMC 2824407. PMID 20080593.
- ↑ Żeromski J, Kaczmarek M, Boruczkowski M, Kierepa A, Kowala-Piaskowska A, Mozer-Lisewska I (June 2019). "Significance and Role of Pattern Recognition Receptors in Malignancy". Archivum Immunologiae et Therapiae Experimentalis. 67 (3): 133–141. doi:10.1007/s00005-019-00540-x. PMC 6509067. PMID 30976817.
- ↑ Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, et al. (August 2000). "Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells". Blood. 96 (4): 1496–1504. doi:10.1182/blood.V96.4.1496. PMID 10942397.
Further reading
- Bowie AG, Fitzgerald KA (April 2007). "RIG-I: tri-ing to discriminate between self and non-self RNA". Trends in Immunology. 28 (4): 147–150. doi:10.1016/j.it.2007.02.002. PMID 17307033.
- Imaizumi T, Aratani S, Nakajima T, Carlson M, Matsumiya T, Tanji K, et al. (March 2002). "Retinoic acid-inducible gene-I is induced in endothelial cells by LPS and regulates expression of COX-2". Biochemical and Biophysical Research Communications. 292 (1): 274–279. doi:10.1006/bbrc.2002.6650. PMID 11890704.
- Cui XF, Imaizumi T, Yoshida H, Borden EC, Satoh K (June 2004). "Retinoic acid-inducible gene-I is induced by interferon-gamma and regulates the expression of interferon-gamma stimulated gene 15 in MCF-7 cells". Biochemistry and Cell Biology. 82 (3): 401–405. doi:10.1139/o04-041. PMID 15181474.
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. (July 2004). "The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses". Nature Immunology. 5 (7): 730–737. doi:10.1038/ni1087. PMID 15208624. S2CID 34876422.
- Imaizumi T, Yagihashi N, Hatakeyama M, Yamashita K, Ishikawa A, Taima K, et al. (July 2004). "Expression of retinoic acid-inducible gene-I in vascular smooth muscle cells stimulated with interferon-gamma". Life Sciences. 75 (10): 1171–1180. doi:10.1016/j.lfs.2004.01.030. PMID 15219805.
- Imaizumi T, Yagihashi N, Hatakeyama M, Yamashita K, Ishikawa A, Taima K, et al. (August 2004). "Upregulation of retinoic acid-inducible gene-I in T24 urinary bladder carcinoma cells stimulated with interferon-gamma". The Tohoku Journal of Experimental Medicine. 203 (4): 313–318. doi:10.1620/tjem.203.313. PMID 15297736.
- Imaizumi T, Hatakeyama M, Yamashita K, Yoshida H, Ishikawa A, Taima K, et al. (2004). "Interferon-gamma induces retinoic acid-inducible gene-I in endothelial cells". Endothelium. 11 (3–4): 169–173. doi:10.1080/10623320490512156. PMID 15370293.
- Sakaki H, Imaizumi T, Matsumiya T, Kusumi A, Nakagawa H, Kubota K, et al. (February 2005). "Retinoic acid-inducible gene-I is induced by interleukin-1beta in cultured human gingival fibroblasts". Oral Microbiology and Immunology. 20 (1): 47–50. doi:10.1111/j.1399-302X.2005.00181.x. PMID 15612946.
- Sumpter R, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, et al. (March 2005). "Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I". Journal of Virology. 79 (5): 2689–2699. doi:10.1128/JVI.79.5.2689-2699.2005. PMC 548482. PMID 15708988.
- Li K, Chen Z, Kato N, Gale M, Lemon SM (April 2005). "Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes". The Journal of Biological Chemistry. 280 (17): 16739–16747. doi:10.1074/jbc.M414139200. PMID 15737993.
- Breiman A, Grandvaux N, Lin R, Ottone C, Akira S, Yoneyama M, et al. (April 2005). "Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon". Journal of Virology. 79 (7): 3969–3978. doi:10.1128/JVI.79.7.3969-3978.2005. PMC 1061556. PMID 15767399.
- Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM (July 2005). "Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways". Proceedings of the National Academy of Sciences of the United States of America. 102 (29): 10200–10205. Bibcode:2005PNAS..10210200Z. doi:10.1073/pnas.0504754102. PMC 1177427. PMID 16009940.
- Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. (September 2005). "Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity". Journal of Immunology. 175 (5): 2851–2858. doi:10.4049/jimmunol.175.5.2851. PMID 16116171.
- Seth RB, Sun L, Ea CK, Chen ZJ (September 2005). "Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3". Cell. 122 (5): 669–682. doi:10.1016/j.cell.2005.08.012. PMID 16125763. S2CID 11104354.
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. (October 2005). "IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction". Nature Immunology. 6 (10): 981–988. doi:10.1038/ni1243. PMID 16127453. S2CID 31479259.
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB (September 2005). "VISA is an adapter protein required for virus-triggered IFN-beta signaling". Molecular Cell. 19 (6): 727–740. doi:10.1016/j.molcel.2005.08.014. PMID 16153868.
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J (October 2005). "Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus" (PDF). Nature. 437 (7062): 1167–1172. Bibcode:2005Natur.437.1167M. doi:10.1038/nature04193. PMID 16177806. S2CID 4391603.
Note: RARRES3 (Gene ID: 5920) and DDX58 (Gene ID: 23586) share the RIG1/RIG-1 alias in common. RIG1 is a widely used alternative name for DExD/H-box helicase 58 (DDX58), which can be confused with the retinoic acid receptor responder 3 (RARRES3) gene, since they share the same alias. [22 Jan 2019]