Uncoordinated-119 (Unc-119) is a protein that has been identified in C. elegans, humans, mice, zebrafish, rabbits, pig, calf, monkey, and protozoa.[5] They have been classified in the GMP phosphodiesterase, delta superfamily.[6] Unc-119 proteins are categorized into their own family but are shown to be ancestrally related to PrBP (prenyl binding protein) and rhoGDI. It has been given many different names: Retinal Protein 4, HRG4, POC7 Centriolar Protein Homolog A, IMD13, POC7A, and RG4.
Structure and function
Unc-119 in C. elegans is approximately 240 amino acids[7] and has a mass of ~26 kDa.[8] Using x-ray crystallography the protein's crystal structure was observed and found to have a resolution of 1.95 Å.[9] It has an immunoglobulin-like β-sandwich folding structure, resulting in a narrow, hydrophobic pocket.[10] This pocket has ability to bind to lauroyl (C12) and myristoyl (C14) acyltransferase side chains as a transporter or lipid-binding chaperone.[9] Unc-119 helps with motility of cilium. It is needed for cilia to maintain formation and function. This is a conserved responsibility from animals to protozoa.
Role in cells
UNC-119 has been found to be involved in synaptic functions, signal transduction, endosome recycling, uptake of bacteria and endocytosis, protein trafficking, lipid-binding chaperone, and a mediator on Src family kinase signals.[11][12]
The UNC-119 protein plays key roles in the movement and feeding in the C. elegans, because it is essential in the development and function of their nervous system. One mutation observed was in the expression patterns when the mutant fuses with lacZ.[10] When this protein is mutated or deleted, C. elegans were found to have problems moving, even to the point of complete paralysis. It was also proposed that a mutation could cause the C. elegans to lose the ability to recognize their food. When the organism possesses a mutated UNC-119, they have been shown to experience uncoordinated movement, a defect causing weak egg laying, and the inability to form dauer larvae.[12]
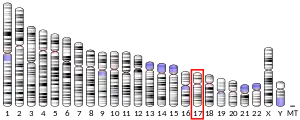
In H. sapiens, Unc-119 has been identified on chromosome 17 and is found predominantly in the retina (HRG4). It has been localized to the photo-receptor synapses in the outer plexiform layer of the retina, and suggested to play a role in the mechanism of photoreceptor neurotransmitter release through the synaptic vesicle cycle. Two transcript variants encoding different isoforms have been described for this gene. The encoded product shares strong homology with the C. elegans unc119 protein and it can functionally complement the C. elegans unc119 mutation.
Unc-119 has also been identified in other areas in humans, such as the liver, kidneys, brain, and fibroblasts.[10] It has also been found to play an important role within the T-cell receptor function[8] and interleukin-5 receptor (IL-5R) Unc-119 is an essential activator of both Lck and Fyn by interacting with their SH2- and SH3-binding domains. Mutation of the Unc-119 gene has been found to severely disrupt the T-cell receptor pathway. It has been suggested to be a cause of an immunodeficiency disorder known as idiopathic CD4 lymphopenia (ICL) due to the reduced t-cell stimulation.[9]
Interactions
Protein unc-119 homolog has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000109103 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000002058 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Swanson DA, Chang JT, Campochiaro PA, Zack DJ, Valle D (October 1998). "Mammalian orthologs of C. elegans unc-119 highly expressed in photoreceptors". Investigative Ophthalmology & Visual Science. 39 (11): 2085–94. PMID 9761287.
- ↑ Fansa EK, Wittinghofer A (October 2016). "Sorting of lipidated cargo by the Arl2/Arl3 system". Small GTPases. 7 (4): 222–230. doi:10.1080/21541248.2016.1224454. PMC 5129900. PMID 27806215.
- ↑ Maduro M, Pilgrim D (November 1995). "Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system". Genetics. 141 (3): 977–88. doi:10.1093/genetics/141.3.977. PMC 1206859. PMID 8582641.
- 1 2 3 4 5 6 Gorska MM, Stafford SJ, Cen O, Sur S, Alam R (February 2004). "Unc119, a novel activator of Lck/Fyn, is essential for T cell activation". The Journal of Experimental Medicine. 199 (3): 369–79. doi:10.1084/jem.20030589. PMC 2211793. PMID 14757743.
- 1 2 3 Constantine R, Zhang H, Gerstner CD, Frederick JM, Baehr W (December 2012). "Uncoordinated (UNC)119: coordinating the trafficking of myristoylated proteins". Vision Research. 75: 26–32. doi:10.1016/j.visres.2012.08.012. PMC 3514684. PMID 23000199.
- 1 2 3 Jean F, Pilgrim D (October 2017). "Coordinating the uncoordinated: UNC119 trafficking in cilia". European Journal of Cell Biology. 96 (7): 643–652. doi:10.1016/j.ejcb.2017.09.001. PMID 28935136.
- ↑ Lee Y, Chung S, Baek IK, Lee TH, Paik SY, Lee J (April 2013). "UNC119a bridges the transmission of Fyn signals to Rab11, leading to the completion of cytokinesis". Cell Cycle. 12 (8): 1303–1315. doi:10.4161/cc.24404. PMC 3674094. PMID 23535298.
- 1 2 Maduro MF, Gordon M, Jacobs R, Pilgrim DB (January 2000). "The UNC-119 family of neural proteins is functionally conserved between humans, Drosophila and C. elegans". Journal of Neurogenetics. 13 (4): 191–212. doi:10.3109/01677060009084494. PMID 10858820. S2CID 36103225.
- ↑ Kobayashi A, Kubota S, Mori N, McLaren MJ, Inana G (January 2003). "Photoreceptor synaptic protein HRG4 (UNC119) interacts with ARL2 via a putative conserved domain". FEBS Letters. 534 (1–3): 26–32. doi:10.1016/S0014-5793(02)03766-3. PMID 12527357. S2CID 22603052.
- ↑ Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- ↑ Van Valkenburgh H, Shern JF, Sharer JD, Zhu X, Kahn RA (June 2001). "ADP-ribosylation factors (ARFs) and ARF-like 1 (ARL1) have both specific and shared effectors: characterizing ARL1-binding proteins". The Journal of Biological Chemistry. 276 (25): 22826–37. doi:10.1074/jbc.M102359200. PMID 11303027.
- 1 2 Cen O, Gorska MM, Stafford SJ, Sur S, Alam R (March 2003). "Identification of UNC119 as a novel activator of SRC-type tyrosine kinases". The Journal of Biological Chemistry. 278 (10): 8837–45. doi:10.1074/jbc.M208261200. PMID 12496276.
- ↑ Alpadi K, Magupalli VG, Käppel S, Köblitz L, Schwarz K, Seigel GM, Sung CH, Schmitz F (September 2008). "RIBEYE recruits Munc119, a mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses". The Journal of Biological Chemistry. 283 (39): 26461–7. doi:10.1074/jbc.M801625200. PMC 3258921. PMID 18664567.
Further reading
- Cen O, Gorska MM, Stafford SJ, Sur S, Alam R (March 2003). "Identification of UNC119 as a novel activator of SRC-type tyrosine kinases". The Journal of Biological Chemistry. 278 (10): 8837–45. doi:10.1074/jbc.M208261200. PMID 12496276.
- Higashide T, Inana G (May 1999). "Characterization of the gene for HRG4 (UNC119), a novel photoreceptor synaptic protein homologous to unc-119". Genomics. 57 (3): 446–50. doi:10.1006/geno.1999.5791. PMID 10329014.
- Kobayashi A, Kubota S, Mori N, McLaren MJ, Inana G (January 2003). "Photoreceptor synaptic protein HRG4 (UNC119) interacts with ARL2 via a putative conserved domain". FEBS Letters. 534 (1–3): 26–32. doi:10.1016/S0014-5793(02)03766-3. PMID 12527357. S2CID 22603052.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (October 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- Swanson DA, Chang JT, Campochiaro PA, Zack DJ, Valle D (October 1998). "Mammalian orthologs of C. elegans unc-119 highly expressed in photoreceptors". Investigative Ophthalmology & Visual Science. 39 (11): 2085–94. PMID 9761287.
- Van Valkenburgh H, Shern JF, Sharer JD, Zhu X, Kahn RA (June 2001). "ADP-ribosylation factors (ARFs) and ARF-like 1 (ARL1) have both specific and shared effectors: characterizing ARL1-binding proteins". The Journal of Biological Chemistry. 276 (25): 22826–37. doi:10.1074/jbc.M102359200. PMID 11303027.
- Vepachedu R, Gorska MM, Singhania N, Cosgrove GP, Brown KK, Alam R (July 2007). "Unc119 regulates myofibroblast differentiation through the activation of Fyn and the p38 MAPK pathway". Journal of Immunology. 179 (1): 682–90. doi:10.4049/jimmunol.179.1.682. PMID 17579091.