Phospholipase D1 (PLD1) is an enzyme that in humans is encoded by the PLD1 gene,[5][6] though analogues are found in plants, fungi, prokaryotes, and even viruses.[7]
History
The possibility of PLD1 was first mentioned in 1947 by authors Hanahan and Chaikoff at Berkeley when describing a carrot enzyme that could "[split] choline from phospholipids."[8] PLD was first derived in mammals in 1975 by Saito and Kanfer, who noted its activity in rats.[9] PLD was first cloned from HeLa cell cDNA in 1995, while mammalian PLD1 was first cloned from a rat in 1997.[7]
Function
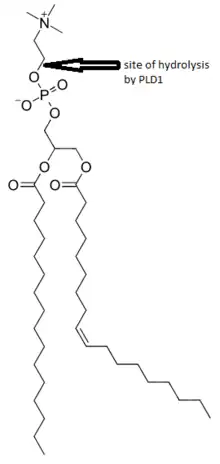
Phosphatidylcholine (PC)-specific phospholipases D (PLDs) EC 3.1.4.4 catalyze the hydrolysis of PC to produce phosphatidic acid (PA) and choline. A range of agonists acting through G protein-coupled receptors and receptor tyrosine kinases stimulate this hydrolysis. PC-specific PLD activity has been implicated in numerous cellular pathways, including membrane trafficking, signal transduction, platelet coagulation, mitosis, apoptosis, and the creation of cytoplasmic lipid droplets.[6][7][10][11]
Membrane trafficking
PLD1 has been shown to associate at the plasma membrane, late endosome,[12] early endosome, and the Golgi apparatus.[7][9] There is evidence that PA is able to assist in negative membrane curvature due to its head group being smaller than in many other lipids.[7] One experiment with PLD1 knockout showed a significant reduction in the number of exocytotic fusion events, implying a strong role in exocytosis.[13]
Signal transduction
PLD1 may play a role in some cells in the endocytosis of signaling receptors or exocytosis of signaling molecules. For example, one experiment in B cells showed that limiting PLD1 led to significantly reduced endocytosis of the B cell receptor.[12] Another experiment showed that knocking out PLD1 may hinder the ability of mice to secrete catecholamines, molecules that are essential for vesicular communication across the body.[13]
Structure
Mammalian PLD1 has several domains for activators, inhibitors, and catalysis, which it shares with PLD2. Domains for both activation and inhibition are referred to as the phox homology (PX) and pleckstrin homology (PH) domains. The catalytic domain consists of two HKD regions, so named for three of the amino acids that are key in catalysis. These domains are conserved across many organisms.[7][9] There are two splice variants of the protein, PLD1a and PLD1b, but they do not seem to localize any differently.[7]
Applications
Alzheimer's disease: PA, which is produced in part by PLD1, seems to be involved in the movement of β-amyloid, which could precede amyloidogenesis.[14]
Cancer: certain rat tumors with dominant negative PLD do not appear to form new colonies or tumors.[7][14]
Thrombosis: PLD knockout mice appear to have reduced occlusion, thus offsetting thrombosis.[7]
Type II Diabetes: the protein PED/PEA15 is often elevated in type II diabetic patients, thus enhancing PLD1 activity, and in turn impairing insulin.[7]
Interactions
Phospholipase D1 has been shown to interact with:
Inhibitors
- Calphostin-c, an inhibitor[7]
- VU-0359595: 1,700-fold selective versus phospholipase D2, IC50 = 3.7nM.[24]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000075651 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000027695 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Park SH, Chun YH, Ryu SH, Suh PG, Kim H (February 1999). "Assignment of human PLD1 to human chromosome band 3q26 by fluorescence in situ hybridization". Cytogenetics and Cell Genetics. 82 (3–4): 224. doi:10.1159/000015105. PMID 9858822. S2CID 46791637.
- 1 2 Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, Engebrecht J, et al. (December 1995). "Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family". The Journal of Biological Chemistry. 270 (50): 29640–3. doi:10.1074/jbc.270.50.29640. PMID 8530346.
- 1 2 3 4 5 6 7 8 9 10 11 Selvy PE, Lavieri RR, Lindsley CW, Brown HA (October 2011). "Phospholipase D: enzymology, functionality, and chemical modulation". Chemical Reviews. 111 (10): 6064–119. doi:10.1021/cr200296t. PMC 3233269. PMID 21936578.
- ↑ Hanahan DJ, Chaikoff IL (April 1947). "The phosphorus-containing lipides of the carrot". The Journal of Biological Chemistry. 168 (1): 233–40. doi:10.1016/S0021-9258(17)35110-4. PMID 20291081.
- 1 2 3 Jenkins GM, Frohman MA (October 2005). "Phospholipase D: a lipid centric review". Cellular and Molecular Life Sciences. 62 (19–20): 2305–16. doi:10.1007/s00018-005-5195-z. PMID 16143829. S2CID 26447185.
- ↑ "Entrez Gene: PLD1 phospholipase D1, phosphatidylcholine-specific".
- ↑ Andersson L, Boström P, Ericson J, Rutberg M, Magnusson B, Marchesan D, et al. (June 2006). "PLD1 and ERK2 regulate cytosolic lipid droplet formation". Journal of Cell Science. 119 (Pt 11): 2246–57. doi:10.1242/jcs.02941. PMID 16723731. S2CID 1628874.
- 1 2 Donaldson JG (September 2009). "Phospholipase D in endocytosis and endosomal recycling pathways". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1791 (9): 845–9. doi:10.1016/j.bbalip.2009.05.011. PMC 2731818. PMID 19540357.
- 1 2 Tanguy E, Costé de Bagneaux P, Kassas N, Ammar MR, Wang Q, Haeberlé AM, et al. (August 2020). "Mono- and Poly-unsaturated Phosphatidic Acid Regulate Distinct Steps of Regulated Exocytosis in Neuroendocrine Cells". Cell Reports. 32 (7): 108026. doi:10.1016/j.celrep.2020.108026. PMID 32814056.
- 1 2 Thakur R, Naik A, Panda A, Raghu P (2019-06-04). "Regulation of Membrane Turnover by Phosphatidic Acid: Cellular Functions and Disease Implications". Frontiers in Cell and Developmental Biology. 7: 83. doi:10.3389/fcell.2019.00083. PMC 6559011. PMID 31231646.
- ↑ Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, et al. (April 2002). "alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells". The Journal of Biological Chemistry. 277 (14): 12334–42. doi:10.1074/jbc.M110414200. PMID 11821392.
- 1 2 Lee C, Kim SR, Chung JK, Frohman MA, Kilimann MW, Rhee SG (June 2000). "Inhibition of phospholipase D by amphiphysins". The Journal of Biological Chemistry. 275 (25): 18751–8. doi:10.1074/jbc.M001695200. PMID 10764771.
- ↑ Walker SJ, Wu WJ, Cerione RA, Brown HA (May 2000). "Activation of phospholipase D1 by Cdc42 requires the Rho insert region". The Journal of Biological Chemistry. 275 (21): 15665–8. doi:10.1074/jbc.M000076200. PMID 10747870.
- ↑ Zhang Y, Redina O, Altshuller YM, Yamazaki M, Ramos J, Chneiweiss H, et al. (November 2000). "Regulation of expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them". The Journal of Biological Chemistry. 275 (45): 35224–32. doi:10.1074/jbc.M003329200. PMID 10926929.
- ↑ Oishi K, Takahashi M, Mukai H, Banno Y, Nakashima S, Kanaho Y, et al. (May 2001). "PKN regulates phospholipase D1 through direct interaction". The Journal of Biological Chemistry. 276 (21): 18096–101. doi:10.1074/jbc.M010646200. PMID 11259428.
- ↑ Luo JQ, Liu X, Hammond SM, Colley WC, Feig LA, Frohman MA, et al. (June 1997). "RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1". Biochemical and Biophysical Research Communications. 235 (3): 854–9. doi:10.1006/bbrc.1997.6793. PMID 9207251.
- ↑ Kim JH, Lee SD, Han JM, Lee TG, Kim Y, Park JB, et al. (July 1998). "Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA". FEBS Letters. 430 (3): 231–5. doi:10.1016/s0014-5793(98)00661-9. PMID 9688545. S2CID 36075513.
- ↑ Genth H, Schmidt M, Gerhard R, Aktories K, Just I (February 2003). "Activation of phospholipase D1 by ADP-ribosylated RhoA". Biochemical and Biophysical Research Communications. 302 (1): 127–32. doi:10.1016/s0006-291x(03)00112-8. PMID 12593858.
- ↑ Cai S, Exton JH (May 2001). "Determination of interaction sites of phospholipase D1 for RhoA". The Biochemical Journal. 355 (Pt 3): 779–85. doi:10.1042/bj3550779. PMC 1221795. PMID 11311142.
- ↑ Lewis JA, Scott SA, Lavieri R, Buck JR, Selvy PE, Stoops SL, et al. (April 2009). "Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity". Bioorganic & Medicinal Chemistry Letters. 19 (7): 1916–20. doi:10.1016/j.bmcl.2009.02.057. PMC 3791604. PMID 19268584.
Further reading
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, et al. (February 1997). "Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha". The Journal of Biological Chemistry. 272 (6): 3860–8. doi:10.1074/jbc.272.6.3860. PMID 9013646.
- Luo JQ, Liu X, Hammond SM, Colley WC, Feig LA, Frohman MA, et al. (June 1997). "RalA interacts directly with the Arf-responsive, PIP2-dependent phospholipase D1". Biochemical and Biophysical Research Communications. 235 (3): 854–9. doi:10.1006/bbrc.1997.6793. PMID 9207251.
- Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, et al. (March 1997). "Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization". Current Biology. 7 (3): 191–201. doi:10.1016/S0960-9822(97)70090-3. PMID 9395408. S2CID 14008613.
- Bae CD, Min DS, Fleming IN, Exton JH (May 1998). "Determination of interaction sites on the small G protein RhoA for phospholipase D". The Journal of Biological Chemistry. 273 (19): 11596–604. doi:10.1074/jbc.273.19.11596. PMID 9565577.
- Lopez I, Arnold RS, Lambeth JD (May 1998). "Cloning and initial characterization of a human phospholipase D2 (hPLD2). ADP-ribosylation factor regulates hPLD2". The Journal of Biological Chemistry. 273 (21): 12846–52. doi:10.1074/jbc.273.21.12846. PMID 9582313.
- Kim JH, Lee SD, Han JM, Lee TG, Kim Y, Park JB, et al. (July 1998). "Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA". FEBS Letters. 430 (3): 231–5. doi:10.1016/S0014-5793(98)00661-9. PMID 9688545. S2CID 36075513.
- Steed PM, Clark KL, Boyar WC, Lasala DJ (October 1998). "Characterization of human PLD2 and the analysis of PLD isoform splice variants". FASEB Journal. 12 (13): 1309–17. doi:10.1096/fasebj.12.13.1309. PMID 9761774. S2CID 27394831.
- Kim JH, Han JM, Lee S, Kim Y, Lee TG, Park JB, et al. (March 1999). "Phospholipase D1 in caveolae: regulation by protein kinase Calpha and caveolin-1". Biochemistry. 38 (12): 3763–9. doi:10.1021/bi982478+. PMID 10090765.
- Kim Y, Han JM, Park JB, Lee SD, Oh YS, Chung C, et al. (August 1999). "Phosphorylation and activation of phospholipase D1 by protein kinase C in vivo: determination of multiple phosphorylation sites". Biochemistry. 38 (32): 10344–51. doi:10.1021/bi990579h. PMID 10441128.
- Walker SJ, Wu WJ, Cerione RA, Brown HA (May 2000). "Activation of phospholipase D1 by Cdc42 requires the Rho insert region". The Journal of Biological Chemistry. 275 (21): 15665–8. doi:10.1074/jbc.M000076200. PMID 10747870.
- Suzuki J, Yamazaki Y, Li G, Kaziro Y, Koide H, Guang L (July 2000). "Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts". Molecular and Cellular Biology. 20 (13): 4658–65. doi:10.1128/MCB.20.13.4658-4665.2000. PMC 85875. PMID 10848592.
- Zhang Y, Redina O, Altshuller YM, Yamazaki M, Ramos J, Chneiweiss H, et al. (November 2000). "Regulation of expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them". The Journal of Biological Chemistry. 275 (45): 35224–32. doi:10.1074/jbc.M003329200. PMID 10926929.
- Divecha N, Roefs M, Halstead JR, D'Andrea S, Fernandez-Borga M, Oomen L, et al. (October 2000). "Interaction of the type Ialpha PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4, 5-bisphosphate in the regulation of PLD2 activity". The EMBO Journal. 19 (20): 5440–9. doi:10.1093/emboj/19.20.5440. PMC 314009. PMID 11032811.
- Oishi K, Takahashi M, Mukai H, Banno Y, Nakashima S, Kanaho Y, et al. (May 2001). "PKN regulates phospholipase D1 through direct interaction". The Journal of Biological Chemistry. 276 (21): 18096–101. doi:10.1074/jbc.M010646200. PMID 11259428.
- Cai S, Exton JH (May 2001). "Determination of interaction sites of phospholipase D1 for RhoA". The Biochemical Journal. 355 (Pt 3): 779–85. doi:10.1042/bj3550779. PMC 1221795. PMID 11311142.
- Lee S, Park JB, Kim JH, Kim Y, Kim JH, Shin KJ, et al. (July 2001). "Actin directly interacts with phospholipase D, inhibiting its activity". The Journal of Biological Chemistry. 276 (30): 28252–60. doi:10.1074/jbc.M008521200. PMID 11373276.
- Hughes WE, Parker PJ (June 2001). "Endosomal localization of phospholipase D 1a and 1b is defined by the C-termini of the proteins, and is independent of activity". The Biochemical Journal. 356 (Pt 3): 727–36. doi:10.1042/0264-6021:3560727. PMC 1221899. PMID 11389680.
- Min DS, Ahn BH, Jo YH (June 2001). "Differential tyrosine phosphorylation of phospholipase D isozymes by hydrogen peroxide and the epidermal growth factor in A431 epidermoid carcinoma cells". Molecules and Cells. 11 (3): 369–78. PMID 11459228.