
Ouchterlony double immunodiffusion (also known as passive double immunodiffusion) is an immunological technique used in the detection, identification and quantification of antibodies and antigens, such as immunoglobulins and extractable nuclear antigens. The technique is named after Örjan Ouchterlony, the Swedish physician who developed the test in 1948 to evaluate the production diphtheria toxins from isolated bacteria.[1]
Procedure
A gel plate is cut to form a series of holes ("wells") in an agar or agarose gel. A sample extract of interest (for example human cells harvested from tonsil tissue) is placed in one well, sera or purified antibodies are placed in another well and the plate left for 48 hours to develop. During this time the antigens in the sample extract and the antibodies each diffuse out of their respective wells. Where the two diffusion fronts meet, if any of the antibodies recognize any of the antigens, they will bind to the antigens and form an immune complex. The immune complex precipitates in the gel to give a thin white line (precipitin line), which is a visual signature of antigen recognition.[2][3]
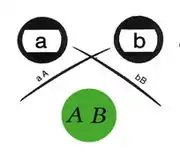
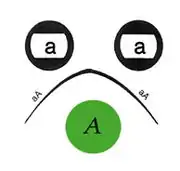
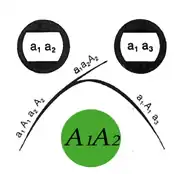
The method can be conducted in parallel with multiple wells filled with different antigen mixtures and multiple wells with different antibodies or mixtures of antibodies, and antigen-antibody reactivity can be seen by observing between which wells the precipitate is observed. When more than one well is used there are many possible outcomes based on the reactivity of the antigen and antibody selected. The zone of equivalence lines may give a full identity (i.e. a continuous line), partial identity (i.e. a continuous line with a spur at one end), or a non-identity (i.e. the two lines cross completely).
The sensitivity of the assay can be increased by using a stain such as Coomassie brilliant blue, this is done by repeated staining and destaining of the assay until the precipitin lines are at maximum visibility.[1]
Theory
Precipitation occurs with most antigens because the antigen is multivalent (i.e. has several antigenic determinants per molecule to which antibodies can bind). Antibodies have at least two antigen binding sites (and in the case of immunoglobulin M there is a multimeric complex with up to 10 antigen binding sites), thus large aggregates or gel-like lattices of antigen and antibody are formed. Experimentally, an increasing amount of antigen is added to a constant amount of antibody in solution. Initially at low antigen concentration, all of the antibody is contained in the precipitate. This is called the antibody-excess zone (i.e. prozone phenomenon). As more antigen is added, the amount of protein precipitated increases until the antigen/antibody molecules are at an optimal ratio. This is known as the zone of equivalence or equivalence point. When the amount of antigen in solution exceeds the amount of antibody, the amount of precipitation will decrease. This is known as the antigen excess zone.
References
- 1 2 Hornbeck, Peter (February 2017). "Double-Immunodiffusion Assay for Detecting Specific Antibodies (Ouchterlony)". Current Protocols in Immunology. 116 (1): 2.3.1–2.3.4. doi:10.1002/cpim.18. PMID 28150863. S2CID 35908711.
- ↑ Moticka, Edward J. (2015). A Historical Perspective on Evidence-Based Immunology. Elsevier. p. 360. ISBN 978-0-12-398381-7.
- ↑ Paul, William E., ed. (2008). "Immunodiffusion and the Ouchterlony Method". Fundamental Immunology (6th ed.). Lippincott Williams & Wilkins. pp. 176–177. ISBN 9780781765190. p. 176 p. 177
- Bailey, Graham S. (1996). "135: Ouchterlony Double Immunodiffusion". In Walker, John M. (ed.). The Protein Protocols Handbook. Springer Protocols Handbooks. Vol. VII: Immunochemical Techniques. Totowa, New Jersey: Humana Press. pp. 749–752. doi:10.1007/978-1-60327-259-9_135. ISBN 978-0-89603-338-2.
- Ouchterlony, Örjan (1948). "In vitro method for testing the toxin-producing capacity of diphtheria bacteria". APMIS. 25 (1–2): 186–191. doi:10.1111/j.1699-0463.1948.tb00655.x. PMID 18887479.
- Ouchterlony, Örjan (1949). "Antigen-antibody reactions in gels". APMIS. 26 (4): 507–515. doi:10.1111/j.1699-0463.1949.tb00751.x. PMID 18143039.
- Ouchterlony, O. (1958). "Diffusion-in-gel methods for immunological analysis". Progress in Allergy. 5: 1–78. PMID 13578996.
- Ouchterlony, O. (1962). "Diffusion-in-gel methods for immunological analysis. II". Progress in Allergy. 6: 30–154. doi:10.1159/000313795. PMID 14482809.
- Ouchterlony, O.; Nilsson, L.A. (1986). Weir, Daniel Mackay (ed.). Handbook of Experimental Immunology. Vol. 1. Immunochemistry (4th ed.). Oxford, England: Blackwell Scientific. pp. 32.1–32.50. ISBN 9780632014996. Retrieved 2012-11-23. at Google Books
- "Ouchterlony Analysis" (PDF). Medical Immunology 544. University of California, Irvine College of Medicine. Fall 2011. Archived from the original (PDF) on 2012-02-07. Retrieved 2012-11-23.
- "Ouchterlony Double Diffusion - Patterns: Theory". Value @ Amrita. India: Amrita Vishwa Vidyapeetham University. 2012. Archived from the original on 2012-11-13. Retrieved 2012-12-24.
- "Ouchterlony Double Diffusion - Patterns: Procedure". Value @ Amrita]. India: Amrita Vishwa Vidyapeetham University. 2012. Archived from the original on 2017-01-14. Retrieved 2012-12-24.
- "Ouchterlony Double Diffusion - Titration: Theory". Value @ Amrita. India: Amrita Vishwa Vidyapeetham University. 2012. Archived from the original on 2012-11-13. Retrieved 2012-11-23.
- "Ouchterlony Double Diffusion - Titration: Procedure". Value @ Amrita. India: Amrita Vishwa Vidyapeetham University. 2012. Archived from the original on 2017-03-16. Retrieved 2012-12-24.
- "BSCI423: Lab 5. Precipitation". College of Computer, Mathematical, and Natural Sciences, University of Maryland, College Park. Archived from the original on 2012-02-05. Retrieved 2012-11-23.
External links
- Simulator at University of California, Irvine
- "Ouchterlony double immunodiffusion" (photograph). Retrieved 2017-05-15. Photograph of Ouchterlony double immunodiffusion plate with unstained precipitin lines of full identity and non-identity.
- "Diffusion Patterns". Immunodiffusion principles and application. Archived from the original on 2019-12-11. Retrieved 2017-05-19. Photographs of Ouchterlony immunodiffusion patterns showing stained precipitin lines of full identity, partial identity and non-identity.
- "Ouchterlony Assay". YouTube. Bio-Rad Laboratories. October 16, 2012.