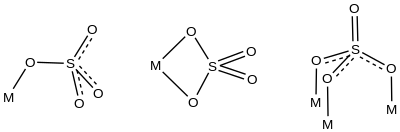
Transition metal sulfate complexes or sulfato complexes are coordination complexes with one or more sulfate ligands. Sulfate binds to metals through one, two, three, or all four oxygen atoms.[1] Common are complexes where sulfate is unidentate or chelating bidentate. Examples are respectively [Co(tren)(NH3)(SO4)]+ (tren = tris(2-aminoethyl)amine)[2] and Co(phen)2SO4.[3] All four oxygen atoms of sulfate bond to metals in some Dawson-type polyoxometalates, e.g. [S2Mo18O62]4-.[4]
Sulfate as a counterion
Being the conjugate base of a strong acid (sulfuric acid), sulfate is not basic. It is more commonly a counterion in coordination chemistry. Tutton's salts, with the formula M'2M(SO4)2(H2O)6 (M' = K+, etc.; M = Fe2+, etc.), illustrate the ability of water to outcompete sulfate as a ligand for M2+. Similarly alums, such as chrome alum ([K(H2O)6][Cr(H2O)6][SO4]2), features [Cr(H2O)6]3+ with noncoordinated sulfate. In a related vein, some sulfato complexes confirmed by X-ray crystallography, convert to simple aquo complexes when dissolved in water. Copper(II) sulfate examplifies this behavior, sulfate is bonded to copper in the crystal but dissociates upon dissolution.
Synthesis
Sulfato complexes are commonly produced by reaction of metal sulfates with other ligands.
In some cases, sulfato complexes are produced from sulfur dioxide:[6]
- Pt(O2)(PPh3)2 + SO2 → Pt(SO4)(PPh3)2 (PPh3 = triphenylphosphine)
Sulfato complexes also arise by air-oxidation of metal sulfides.[7]
Reactions
Sulfato complexes are susceptible to protonation of uncoordinated oxygen atoms.[8]
References
- ↑ Papatriantafyllopoulou, Constantina; Aromi, Guillem; Tasiopoulos, Anastasios J.; Nastopoulos, Vassilios; Raptopoulou, Catherine P.; Teat, Simon J.; Escuer, Albert; Perlepes, Spyros P. (2007). "Use of the Sulfato Ligand in 3d‐Metal Cluster Chemistry: A Family of Hexanuclear Nickel(II) Complexes with 2‐Pyridyl‐Substituted Oxime Ligands". European Journal of Inorganic Chemistry. 2007 (18): 2761–2774. doi:10.1002/ejic.200700063.
- ↑ Chun, Hyungphil; Jackson, W. Gregory; McKeon, Josephine A.; Somoza, Fernando B.; Bernal, Ivan (2000). "The Interaction between Amminehalocobalt(III) Cations and Polythionate Anions: Hydrogen-Bonding Patterns and S–S Bond Cleavage Reactions". European Journal of Inorganic Chemistry. 2000: 189–193. doi:10.1002/(SICI)1099-0682(200001)2000:1<189::AID-EJIC189>3.0.CO;2-V.
- ↑ Zhong, Kai-Long (2013). "Bis(1,10-phenanthroline-κ2 N , N ′)(sulfato-κ2 O , O ′)cobalt(II) propane-1,2-diol monosolvate". Acta Crystallographica Section E. 69 (Pt 1): m26. doi:10.1107/S1600536812049616. PMC 3588351. PMID 23476325.
- ↑ Juraja, Suzy; Vu, Truc; Richardt, Peter J. S.; Bond, Alan M.; Cardwell, Terence J.; Cashion, John D.; Fallon, Gary D.; Lazarev, Georgii; Moubaraki, Boujemaa; Murray, Keith S.; Wedd, Anthony G. (2002). "Electrochemical, Spectroscopic, Structural, and Magnetic Characterization of the Reduced and Protonated α-Dawson Anions in [Fe(η5-C5Me5)2]5[HS2Mo18O62]·3HCONMe2·2Et2O and [NBu4]5[HS2Mo18O62]·2H2O1". Inorganic Chemistry. 41 (5): 1072–1078. doi:10.1021/ic010780s. PMID 11874340.
- ↑ Schaniel, Dominik; Woike, Theo; Behrnd, Norwid-R.; Hauser, Jürg; Krämer, Karl W.; Todorova, Teodora; Delley, Bernard (2009). "Photogeneration of Nitrosyl Linkage Isomers in Octahedrally Coordinated Platinum Complexes in the Red Spectral Range". Inorganic Chemistry. 48 (23): 11399–11406. doi:10.1021/ic901392q. PMID 19863116.
- ↑ Cook, Christopher David.; Jauhal, G. S. (1967). "Oxidation of Coordinated Ligands. Sulfato and Nitrato Complexes of Platinum". Journal of the American Chemical Society. 89 (12): 3066–3067. doi:10.1021/ja00988a057.
- ↑ Kim, Chang G.; Coucouvanis, Dimitri (1993). "Dimerization of the molybdenum complex[(SO4)Mo(O)(mu-S)2Mo(O)(SO4)]2- Dianions Stabilized by a Quadruply Bridging Sulfate Ligand and Intramolecular Hydrogen Bonding by the [(CH3)2NH2]+ Cations. Structures of the [(CH3)2NH2]6-x(Et4N)x[(SO4)Mo(O)(mu-S)2Mo(O)(SO4)]2(SO4)] Aggregates (x = 1, 2, 2.5)". Inorganic Chemistry. 32 (11): 2232–2233. doi:10.1021/ic00063a004.
- ↑ Scheidt, W. Robert; Lee, Young Ja; Finnegan, Michael G. (1988). "Reactions of Sulfur Dioxide with Iron Porphyrinates and the Crystal Structure of (Hydrogen sulfato)(tetraphenylporphinato)iron(III) Hemibenzene Solvate". Inorganic Chemistry. 27 (26): 4725–4730. doi:10.1021/ic00299a010.