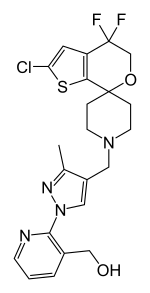
 | |
| Clinical data | |
|---|---|
| Other names | BTRX-246040 |
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H23ClF2N4O2S |
| Molar mass | 480.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
BTRX-246040, also known as LY-2940094, is a potent and selective nociceptin receptor antagonist which is under development by BlackThorn Therapeutics and Eli Lilly for the treatment of major depressive disorder (MDD).[1][2][3] It has demonstrated proof-of-concept clinical efficacy for depression.[4] As of 2017, it is in phase II clinical trials for the treatment of MDD.[1][2][3] It was also under investigation for the treatment of alcoholism, and similarly reached phase II clinical studies for this indication, but development was discontinued.[1]
See also
References
- 1 2 3 "BTRX 246040". AdisInsight. Springer Nature Switzerland AG.
- 1 2 Dale E, Bang-Andersen B, Sánchez C (May 2015). "Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs". Biochemical Pharmacology. 95 (2): 81–97. doi:10.1016/j.bcp.2015.03.011. PMID 25813654.
- 1 2 Yin X, Guven N, Dietis N (2015). "Opioids in Depression: Not Quite There Yet". UK Journal of Pharmaceutical and Biosciences. 3 (1): 12–17. doi:10.20510/ukjpb/3/i1/89219.
- ↑ Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, et al. (June 2016). "A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies". Neuropsychopharmacology. 41 (7): 1803–1812. doi:10.1038/npp.2015.348. PMC 4869049. PMID 26585287.
External links
- "BTRX-246040 (LY-2940094)". AdisInsight. Springer Nature Switzerland AG.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.