The Herz reaction, named after the chemist Richard Herz, is the chemical conversion of an aniline to the benzodithiazolium salt by its reaction with disulfur dichloride. The salt is called a Herz salt. Hydrolysis of this Herz salt give the corresponding sodium thiolate, which can be further converted to the 2-aminothiophenol.[1]
.png.webp)
The 2-aminothiophenols are suitable for diazotization, giving benzothiadiazoles.[2] Instead the sodium 2-aminothiophenolate can be converted to a 1,3-benzothiazole.
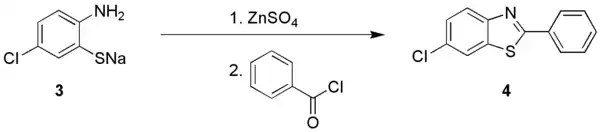 Herz-reaction application
Herz-reaction application
Dyes
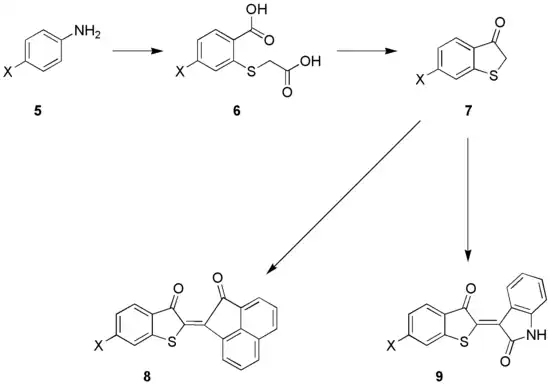
Aniline 5 is converted to compound 6, in three steps;
- conversion to an ortho-aminothiol through the Herz-reaction (aniline 5 and disulfur dichloride), followed by
- conversion to an ortho-aminoarylthioglycolacid and
- conversion of the aromatic amine function to a nitrile via the Sandmeyer reaction.
- In a last step the nitrile is hydrolysed resulting in 6. This compound is converted to 7 via a ring-closing reaction and decarboxylation.
The compound, (thioindoxyl, 7) is an important intermediate in the organic synthesis of some dyes. Condensation with acenaphthoquinone gives 8, a dye of the so-called Ciba-Scarlet type, while condensation of 7 with isatin results in the thio-Indigo dye 9.
References
- ↑ W. K. Warburton (1957). "Arylthiazathiolium Salts And o-Aminoaryl Thiols - The Herz Reaction". Chemical Reviews. 57 (5): 1011–1020. doi:10.1021/cr50017a004.
- ↑ Kirby, P.; Soloway, S. B.; Davies, J. H.; Webb, Shirley B. (1970). "1,2,3-Benzothiadiazoles. Part I. A simplified synthesis of 1,2,3-benzothiadiazoles". Journal of the Chemical Society C: Organic (16): 2250. doi:10.1039/J39700002250.