 | |
 | |
| Names | |
|---|---|
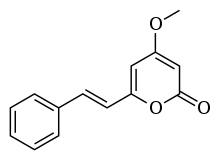
| Preferred IUPAC name
4-Methoxy-6-[(E)-2-phenylethen-1-yl]-2H-pyran-2-one | |
| Other names
(E)-4-Methoxy-6-styryl-2H-pyran-2-one 5,6-Dehydrokavain 4-Methoxy-6-[(E)-2-phenylvinyl]-2-pyranone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | white to faint yellow powder |
| Density | 1.18 g/mL |
| Melting point | 148 °C (298 °F; 421 K) |
| Boiling point | 440 °C (824 °F; 713 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Desmethoxyyangonin or 5,6-dehydrokavain is one of the six main kavalactones found in the Piper methysticum (kava) plant.
Pharmacology
Desmethoxyyangonin is a reversible inhibitor of monoamine oxidase B (MAO-B).[1] Kava is able to increase dopamine levels in the nucleus accumbens[2] and desmethoxyyangonin likely contributes to this effect. This, along with several other catecholamines, may be responsible for the purported attention-promoting effects of kava.
Unlike the other major kavalactones, desmethoxyyangonin does not appear to act as a GABAA receptor positive allosteric modulator.[3]
Desmethoxyyangonin has marked activity on the induction of CYP3A23.[4]
See also
References
- ↑ Uebelhack, R; Franke L; Schewe HL (September 1998). "Inhibition of platelet MAO-B by kava pyrone-enriched extract from Piper methysticum Forster (kava-kava)". Pharmacopsychiatry. 31 (5): 187–192. doi:10.1055/s-2007-979325. PMID 9832350. S2CID 25270815.
- ↑ Baum, SS; Hill R; Rommelspacher H (October 1998). "Effect of kava extract and individual kavapyrones on neurotransmitter levels in the nucleus accumbens of rats". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 22 (7): 1105–1120. doi:10.1016/S0278-5846(98)00062-1. PMID 9829291. S2CID 24377397.
- ↑ Boonen, G.; Häberlein, H. (1998). "Influence of genuine kavapyrone enantiomers on the GABA-A binding site". Planta Medica. 64 (6): 504–506. doi:10.1055/s-2006-957502. PMID 9776662. S2CID 45511040.
- ↑ Ma, Yuzhong; Karuna Sachdeva; Jirong Liu1; Michael Ford; Dongfang Yang; Ikhlas Khan; Clinton Chichester; Bingfang Yan (November 2004). "Desmethoxyyangonin and dihydromethysticin are two major pharmacological kavalactones with marked activity on the induction of CYP3A23". Drug Metabolism and Disposition. 32 (11): 1317–1324. doi:10.1124/dmd.104.000786. PMID 15282211. S2CID 43840844.
{{cite journal}}: CS1 maint: numeric names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.