| Anoplotherium | |
|---|---|
 | |
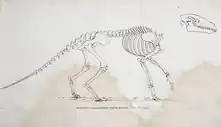
| Anoplotherium commune incomplete skeleton from the commune of Pantin, National Museum of Natural History, France | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Artiodactyla |
| Family: | †Anoplotheriidae |
| Subfamily: | †Anoplotheriinae |
| Genus: | †Anoplotherium Cuvier, 1804 |
| Type species | |
| †Anoplotherium commune Cuvier, 1804 | |
| Other species | |
| |
| Synonyms | |
|
Genus synonymy
Synonyms of A. commune
Synonyms of A. latipes
Synonyms of A. laurillardi
| |
Anoplotherium is the type genus of the extinct Paleogene artiodactyl family Anoplotheriidae, which was endemic to western Europe. It lived from the late Eocene to the earliest Oligocene. It was the fourth fossil mammal genus to be described with official taxonomic authority, with a history extending back to 1804 when its fossils from Montmartre in Paris, France were first described by the French naturalist Georges Cuvier. Discoveries of incomplete skeletons of A. commune in 1807 led Cuvier to thoroughly describe unusual features for which there are no modern analogues. His drawn skeletal and muscle reconstructions of A. commune in 1812 were amongst the first instances of anatomical reconstructions based on fossil evidence. Cuvier's contributions to palaeontology based on his works on the genus were revolutionary for the field, not only proving the developing ideas of extinction and ecological succession but also paving the way for subfields such as palaeoneurology. Today, there are four known species.
Anoplotherium was amongst the largest non-whippomorph artiodactyls of the Paleogene period, weighing on average 115 kg (254 lb) to 271 kg (597 lb) and measuring at least 2.5 m (8 ft 2 in) in head and body length and 1.25 m (4 ft 1 in) in shoulder height. It was an evolutionarily advanced and unusual artiodactyl, sporting three-toed feet in certain species like A. latipes, a long and robust tail, and a highly-developed brain with strong support for both sense of smell and sensory perception. Its overall robust build may have allowed it to stand bipedally to browse on plants at greater heights, reaching approximately 3 m (9.8 ft) tall, effectively competing with the few other medium to large herbivores it lived with. The full extent of its bipedalism needs to be confirmed by more research, however. The larger, two-toed A. commune and slightly smaller, three-toed A. latipes may be sexual dimorphs in that the former is female and the latter male, but this idea remains speculative.
The artiodactyl lived in western Europe back when it was an archipelago that was isolated from the rest of Eurasia, meaning that it lived in an environment with various other faunas that also evolved with strong levels of endemism. Its exact origins are unknown, but it arose long after a shift towards drier but still subhumid conditions that led to abrasive plants and the extinctions of the large-sized Lophiodontidae, achieving gigantism and establishing itself as a dominant herbivore throughout the entirety of the western European region given its abundant fossil evidence.
Its success was abruptly halted by the Grande Coupure extinction and faunal turnover event in the earliest Oligocene of western Europe, which was caused by shifts towards further glaciation and seasonality. Tropical and subtropical forests were rapidly replaced by more temperate environments, and most ocean barriers previously separating western Europe from eastern Eurasia closed, allowing for large faunal dispersals from Asia. Although the specific causes are uncertain, Anoplotherium was likely unable to adapt to these major changes and succumbed to extinction.
Research history
Identifications


While Georges Cuvier knew about fossil bones from the gypsum quarries of the outskirts of Paris (known as the Paris Basin) as early as at least 1800, it was not until 1804 that he would describe them. After describing Palaeotherium, he wrote about the next set of fossils that he was able to discern as being different from Palaeotherium based on dentition form, including the apparent lack of canines that left a large gap between the incisors and premolars. He observed that the hemimandible (half a mandible) had three lower incisors instead of four incisors or none which he said characterized other "pachyderms". Cuvier, basing the name on its apparent lack of suitable arms and canines for offensive attacks, erected the name Anoplotherium.[1][2]
The genus name Anoplotherium means 'unarmed beast', and is a compound of the Greek words αν- (an, 'not'), ὅπλον (hóplon, 'armor, large shield'), and θήρ (thēr, 'beast, wild animal').[3]
Cuvier named three species of Anoplotherium in the same year, the first of which was the "sheep-sized" A. commune and the other three of which were "smaller species" that he named A. medium, A. minus, and A. minimum. The etymology of the species name A. commune refers to how "common" fossils of the species were while the etymologies of the other two species were based on sizes compared to A. commune.[lower-alpha 1] He also attributed a cloven hoof (or didactyl hoof) to A. commune since the specimen appeared to be large-sized. He thought that Anoplotherium had didactyl hooves instead of tridactyl hooves, which would have separated it from Palaeotherium. Based on the hooves and dentition, he concluded that Anoplotherium was similar to ruminants or camelids.[4][5] However, in 1807, Cuvier found out that Anoplotherium commune had three toes on its hind limbs, although the third index toes were of smaller sizes compared to the other two.[6]
Skeletons
In 1807, Cuvier wrote about two incomplete skeletons that were recently uncovered, although the first was partially damaged because it was not collected carefully (which he expressed as having frustrated his understanding of the skeletal anatomy of Anoplotherium initially). The first skeleton, found in the quarries of Montmartre in the commune of Pantin, helped to confirm Cuvier's earlier diagnoses of Anoplotherium as correct. The embedded skeleton was the size of a small horse and helped to confirm the large didactyl feet and the 44 total teeth that it had (11 in each side of its jaw). It also had 11 complete ribs and a fragment of a 12th, matching with the number of ribs of camelids. The most surprising element to Cuvier, however, was the enormous tail with 22 vertebrae in the skeleton, a feature that he said he would not have known about previously, as there are no modern analogues of the elongated and thick tail in any large quadrupedal mammal.[7]
The second incomplete skeleton came from Antony, this time more carefully removed with supervision from experts than the first skeleton. In it, he was able to confirm six lumbar vertebrae and three sacral vertebrae, all of which were extremely strong and probably supported the long tail. Most notable to Cuvier was the confirmation that Anoplotherium had two large fingers and one small finger on its front legs, which was unusual for mammals related to it.[7]
Significance in palaeontological history

Although Palaeotherium and Anoplotherium are not well-recognized compared to fossil animals of other periods (ie. Mesozoic dinosaurs and Neogene-Quaternary mammals), their fossil discoveries in Montmartre and formal descriptions by Cuvier are recognized as critical moments that pioneered palaeontology to the modern era. Unlike Pleistocene fossil genera in the Americas in early palaeontological history such as Megatherium and Mammut, Palaeotherium and Anoplotherium were not found in surface-level deposits but embedded in deeper, harder rock deposits dating to the Eocene. People in Paris had been previously familiar with animal skeletons being in their area for centuries, some of which were later kept and formally described. However, it was Cuvier who formally erected two fossil genera that came from older deposits, and from his homeland in the continent of Europe instead of the Americas where Megatherium and Mammut were found.[8] The Paleogene-aged fossils left no evidence of any later descendants, extinct or extant, although the similarities of Palaeotherium to tapirs made proving the theory more difficult. He noticed that below the gypsum was older sediments of seashells and reptiles like what Cuvier described as a giant "crocodile", which would later be known as Mosasaurus. Cuvier knew then that the world that Anoplotherium and Palaeotherium came from was a different span of time before that of the preceding time of sea reptiles and the proceeding times of Megatherium and Mammut, thereby proving the concept of natural extinction.[9]
Cuvier's descriptions of an endocast (fossilized brain case) of a cerebral hemisphere belonging to a broken skull of A. commune from Montmartre, starting from 1804 up to 1822, are recognized as the first true instance of palaeoneurology, the study of brain evolution. The very first definition of an "endocast" dates back to 1822 when Cuvier described a mould of the brain of A. commune, noticing that it offered hints to the true shape of the brain of the now-extinct mammal (although it was later found to be a portion of the brain rather than the entirety of it). Since the first endocast study, many other brain studies were conducted for other fossil mammals throughout the second half of the 19th century onward.[10][11][12] An 1822 description by Cuvier of a healed fractured femur of A. commune is cited as an early instance of palaeopathology, the study of ancient diseases and injuries on prehistoric organisms.[13][14][15]
Early depictions
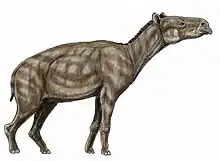
In 1812, Cuvier published his drawing of a skeletal reconstruction of A. commune based on known fossil remains of the species including the aforementioned incomplete skeletons. Based on the robust build of the mammal species, he hypothesized that its body structure was similar to otters except for its legs, that it was adapted for semi-aquatic life by swimming for consumption of aquatic plants, lacking long ears similar to semi-aquatic mammals, and living in marshy environments. Cuvier suggested that its lifestyle was therefore similar to semi-aquatic quadrupedal mammals like hippopotamuses and muroid rodents. He thought that in comparison, other species of Anoplotherium such as A. medium and A. minus were adapted for terrestrial behaviours and mixed feeding (browsing and grazing).[16][17] Today, the reconstruction for the skeletal anatomy has aged well, mostly standing the test of time since 1812.[18] Anoplotherium and Palaeotherium were also depicted in 1822 drawings by the French palaeontologist Charles Léopold Laurillard under the direction of Cuvier, although the restorations were not as detailed as Cuvier's.[19]
The reconstruction of Anoplotherium as an aquatic swimmer was supported by multiple 19th century European palaeontologists and persisted for over a century[20][21] until 1938 when M. Dor rejected the theory of the genus as being aquatic-adapted based on anatomical differences from otters and hippopotamuses that contradict semi-aquatic behaviours and are more consistent with terrestrial life.[22] This rejection was supported by Jerry J. Hooker in 2007 and Svitozar Davydenko et. al. in 2023 based on anatomical traits, although the former disagreed with Dor's observations on the tail. Hooker argued that although the distal caudal vertebrae of the anoplothere are less prominent than those of kangaroos (Macropus), the vertebrae patterns of Anoplotherium are more similar to Macropus than ungulates like Bos or Equus. Today, Anoplotherium is thought to be a terrestrial browser with specialized behaviours.[23][24]

A. commune was notably depicted in the Crystal Palace Dinosaurs attraction in the Crystal Palace Park in the United Kingdom, open to the public since 1854 and constructed by English sculptor Benjamin Waterhouse Hawkins. More specifically, three statues of A. commune were made, two of which are standing and the third of which is in a reposed position. These statues resemble hybrids of deer and big cats and measure 3.6 m (12 ft) long. Its inclusion in the Crystal Palace Park reflects the popularity and public interest in Anoplotherium in the 19th century, as it was an icon of palaeontology, geology, and natural history that it was regularly incorporated in palaeontological texts and classrooms (its popularity diminished since the 20th century).[25][26]
The sculptures of A. commune were overall based on Hawkins closely following Cuvier's description of the genus based on known remains, including Cuvier's unpublished robust muscle speculations which are seen as accurate by modern-day standards. Hawkins did also deviate outside of Cuvier's descriptions, however, likely basing its facial designs and the inaccurate presence of tetradactyl limbs (four toes on each foot) instead of didactyl or tridactyl limbs on extant camelids. Besides these errors, the statues have largely been accurate to modern-day depictions of Anoplotherium.[26]
Confusions with other mammal groups
.jpg.webp)
For much of the 19th century, palaeontologists confused mammals of other families with Anoplotherium largely due to palaeontology being at its early stages. One of the earlier examples is 1822, when Cuvier erected the names A. gracile, A. murinus, A. obliquum, A. leporinum, and A. secundaria, replacing earlier species names within Anoplotherium outside of A. commune. In A. gracile, he noticed differences in the molars that he erected the subgenus Xiphodon. For A. leporinum, the subgenus Dichobune was created by him based on its small size.[11] In 1852, Paul Gervais promoted the 2 subgenera to genus ranks and erected an additional genus Amphimeryx for A. murinus. Therefore, the species are no longer classified as Anoplotherium but distant genera.[27][28]
Other mammals initially confused with the genus Anoplotherium but eventually reclassified within the 19th century represented the endemic European artiodactyl family Cainotheriidae (Cainotherium[29][30][28][31]), European and Indian subcontinental members of the perissodactyl family Chalicotheriidae (Anisodon[32][33][34] and Nestoritherium[35][36][37]), and even endemic South American members of the order Litopterna (Scalabrinitherium and Proterotherium[38]).
Revisions within the Anoplotheriidae

In 1851, Pomel observed that Anoplotherium species could be determined as having either didactyl hooves (lessened third index) or tridactyl hooves (greater-developed third index) and that the only previously erected species that are valid are A. commune and A. secundaria. In addition, he erected three new species based on additional remains: A. duvmoyi (based on Cuvier's fossil illustrations of A. commune), A. platypus, A. laurillardi (convex incisors on the anterior surface), and A. cuvieri. A. laurillardi derives as a species name from Charles Laurillard.[30]
Gervais in 1852 named the genus Eurytherium based on its presence of tridactyl hooves instead of didactyl hooves, for he made the new species E. latipis the type species and A. platypus a synonym of the former.[20] Henri Filhol would follow Gervais by erecting E. quercyi and E. minus based on dental sizes and reclassifying A. secundarium (or A. secundaria) to Eurytherium.[39]
In 1862, Ludwig Rütimeyer erected the subgenus Diplobune for the genus Dichobune on the basis that it was an evolutionary transition between Anoplotherium secundarium and the dichobunid.[40] It was promoted to a distinct genus with one species D. bavaricum being placed into the genus by Oscar Fraas by 1870, however.[41]
In 1883, Max Schlosser made Eurytherium a synonym of Anoplotherium because he argued that the limb anatomies and dentitions were specific differences in characteristics rather than major ones that defined an entire genus. Sclosser pointed out that all species of Anoplotherium in some form had three indexes despite A. commune having less developed third indexes than A. latipes. He also reinforced the idea that "A. platypus" is a synonym of A. latipes. The name A. latipes takes priority over A. platypus to the modern day because Pomel in 1851 did not list any specimen for the species, effectively making it a nomen dubium. He also mentioned that the status of A. duvmoyi was not stable due to being based on illustrations, which he considered to be a "hopeless effort". He also supported Diplobune being a valid genus in that he argued that A. secundaria should be renamed to D. secundaria based on dentition and smaller sizes. Schlosser also said that A. cuvieri was an invalid species because the diagnosis based on isolated metatarsal bones was valid-enough.[21][28][23]
Richard Lydekker erected the species A. cayluxense in 1885 based on its smaller size and unique variations in the molar cusps. He also demoted the genus Diplobune as a synonym of Anoplotherium, meaning that the former's species were added/readded to Anoplotherium as A. secundarium, A. quercyi, A. modicum, A. bavaricum, and A. minus (= A. minor, Filhol 1877).[28] The synonymy of Diplobune with Anoplotherium was not supported by Hans Georg Stehlin in 1910, as he argued that the former was generically distinct from the latter despite their close relations, thus restoring the previous species into Diplobune (with the exception of D. modicum, which he synonymized with D. bavarica) and adding "A. secundarium" into Diplobune as D. secundaria. He also wrote that A. cayluxense was a synonym of D. secundaria. As a result of the revisions, the only valid species of Anoplotherium were A. commune, A. latipes, and A. laurillardi.[42]
In 1922, Wilhelm Otto Dietrich erected the fourth species A. pompeckji from the locality of Mähringen in Germany, named in honor of German palaeontologist Josef Felix Pompeckj. The species was described as a medium-sized tridactyl species with 4-fingered front limbs and 3-toed hind limbs with slimmer hand bone proportions and a smaller astragalus.[43] A. pompeckji is the least characterized species and has similar dentition to A. laurillardi, making its status less certain compared to the three other species.[23][44]
In 1964, palaeontologist Louis de Bonis reviewed briefly the taxonomic synonyms of Anoplotherium, considering that A. duvernoyi was based on a young individual with incisor characteristics that Pomel did not specify and that A. cuvieri does not differ in metacarpral dimensions from A. laurillardi. He followed Stehlin in recognizing the three main species of Anoplotherium, although he did not mention A. pompeckji in his review.[45]
Classification

Anoplotherium is the type genus of the Anoplotheriidae, a Paleogene artiodactyl family endemic to western Europe that lived from the middle Eocene to the early Oligocene (~44 to 30 Ma, possible earliest record at ~48 Ma). The exact evolutionary origins and dispersals of the anoplotheriids are uncertain, but they exclusively resided within the continent when it was an archipelago that was isolated by seaway barriers from other regions such as Balkanatolia and the rest of eastern Eurasia. The Anoplotheriidae's relations with other members of the Artiodactyla are not well-resolved, with some determining it to be either a tylopod (which includes camelids and merycoidodonts of the Paleogene) or a close relative to the infraorder and some others believing that it may have been closer to the Ruminantia (which includes tragulids and other close Paleogene relatives).[46][44]
The Anoplotheriidae consists of two subfamilies, the Dacrytheriinae and Anoplotheriinae, the latter of which is the younger subfamily that Anoplotherium belongs to. The Dacrytheriinae is the older subfamily of the two that first appeared in the middle Eocene (since the Mammal Paleogene zones unit MP13, possibly up to MP10), although some authors consider them to be a separate family in the form of the Dacrytheriidae.[47][48] Anoplotheriines made their first appearances by the late Eocene (MP15-MP16), or ~41-40 Ma, within western Europe with Duerotherium and Robiatherium. By MP17a-MP17b, however, there is a notable gap in the fossil record of anoplotheriines overall as the former two genera seemingly made their last appearances by the previous MP level MP16.[49]
By MP18, Anoplotherium and Diplobune made their first appearances in western Europe, but their exact origins are unknown. The two genera were widespread throughout western Europe based on abundant fossil evidence spanning from Portugal, Spain, United Kingdom, France, Germany, and Switzerland for much of pre-Grande Coupure Europe (prior to MP21), meaning that they were typical elements of the late Eocene up until the earliest Oligocene.[50][49][44] The earlier anoplotheriines are considered to be smaller species whereas the later anoplotheriines were larger. Anoplotherium and Diplobune are considered the most derived (or evolutionarily recent) anoplotheriids based on dental morphology and achieved gigantism amongst non-whippomorph artiodactyls, making them some of the largest non-whippomorph artiodactyls of the Paleogene as well as amongst the largest mammals to roam western Europe at the time (all species of Anoplotherium were large to very large whereas not all species of Diplobune were large).[44][51][52][12]

Conducting studies focused on the phylogenetic relations within the Anoplotheriidae has proven difficult due to the general scarcity of fossil specimens of most genera.[49] The phylogenetic relations of the Anoplotheriidae as well as the Xiphodontidae, Mixtotheriidae, and Cainotheriidae have also been elusive due to the selenodont morphologies of the molars, which were convergent with tylopods or ruminants.[52] Some researchers considered the selenodont families Anoplotheriidae, Xiphodontidae, and Cainotheriidae to be within Tylopoda due to postcranial features that were similar to the tylopods from North America in the Paleogene.[23] Other researchers tie them as being more closely related to ruminants than tylopods based on dental morphology. Different phylogenetic analyses have produced different results for the "derived" selenodont Eocene European artiodactyl families, making it uncertain whether they were closer to the Tylopoda or Ruminantia.[53][54]
In an article published in 2019, Romain Weppe et. al. conducted a phylogenetic analysis on the Cainotherioidea within the Artiodactyla based on mandibular and dental characteristics, specifically in terms of relationships with artiodactyls of the Paleogene. The results retrieved that the superfamily was closely related to the Mixtotheriidae and Anoplotheriidae. They determined that the Cainotheriidae, Robiacinidae, Anoplotheriidae, and Mixtotheriidae formed a clade that was the sister group to the Ruminantia while Tylopoda, along with the Amphimerycidae and Xiphodontidae split earlier in the tree.[54] The phylogenetic tree used for the journal and another published work about the cainotherioids is outlined below:[55]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In 2022, Weppe created a phylogenetic analysis in his academic thesis regarding Paleogene artiodactyl lineages, focusing most specifically on the endemic European families. The phylogenetic tree, according to Weppe, is the first to conduct phylogenetic affinities of all anoplotheriid genera, although not all individual species were included. He found that the Anoplotheriidae, Mixtotheriidae, and Cainotherioidea form a clade based on synapomorphic dental traits (traits thought to have originated from their most recent common ancestor). The result, Weppe mentioned, matches up with previous phylogenetic analyses on the Cainotherioidea with other endemic European Paleogene artiodactyls that support the families as a clade. As a result, he argued that the proposed superfamily Anoplotherioidea, composing of the Anoplotheriidae and Xiphodontidae as proposed by Alan W. Gentry and Hooker in 1988, is invalid due to the polyphyly of the lineages in the phylogenetic analysis. However, the Xiphodontidae was still found to compose part of a wider clade with the three other groups. Anoplotherium and Diplobune compose a clade of the Anoplotheriidae because of their derived dental traits, supported by them being the latest-appearing anoplotheriids.[52][56]
Description
Skull

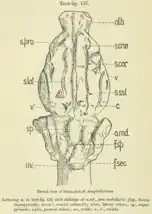
The Anoplotheriidae is characterized in part by low-proportioned skulls with elongated muzzles (the muzzle aligns with the top of the cranium in the case of Anoplotherium), and a wide-open skull orbit.[57][47][44] Anoplotherium lacks bony processes and lacrimal fossae. It has large paroccipital processes and shorter postorbital process projections of the lacrimal bone.[58][59]
The skull of Anoplotherium is narrow and elongated, with a constricted postorbital bone indicating poor brain development. It features robust sagittal and nuchal crests, the former having high elevations and emerging from low postorbital ridges and the latter having complicated elevation shifts. The back has a circular foramen magnum and large occipital condyles. The underside has an elongated palate with glenoid surfaces and strong post-glenoid processes of the squamosal bone.[60]
The skull's bones are robust, with the spongy diploë bone being greatly developed. The skull's strength is attributed to massive temporal muscles as part of an overall strong body build. The skull has a shallow sella turcica, a pear-shaped cranial fossa, extensive parietal bones, large squamosal bone, narrow occipital bone, and two small occipital buns for muscle attachment. Many cranial traits seen in Anoplotherium are also found in the closely related Diplobune.[60][61]
In the auditory region (including the temporal bones), the periotic bone of the inner ear is extensive, the internal auditory meatus and facial canal openings of the temporal bone being visible in the lower triangular area of the periotic bone. The tympanic part of the temporal bone is connected partially to the squamosal bone, remains separate from the periotic bone, and consists of a small but thick auditory bulla (hollow bony structure of the auditory region), which projects underneath the petrous part of the temporal bone.[60]
In a skull fragment of A. laurillardi with incisors and canine alveoli, the known length of the nasal region is large, measuring 38.1 mm (1.50 in).[44] The trait of large nasals is similar to what was observed in a skull of Diplobune secundaria, which are recorded to be massive, elongated, and connected to each other and the maxilla. Cyril Gagnaison and Jean-Jacques Leroux proposed in the case of D. secundaria that the elongated nasal region supports the presence of a very tapered tongue, which similar to giraffes may have allowed it to pull plant branches.[62]
Endocast anatomy
In 1913, R.W. Palmer conducted studies on the brain cast from a cranium of Anoplotherium commune, originating from the Phosphorites of Quercy within the British Museum collections (the endocast is now in the National Museum of Natural History, France as the specimen BMNH 3753). The individual in question was estimated to have weighed 80 kg (180 lb) by its death similar to extant llamas, weighing considerably less than typical estimates of adult Anoplotherium. The total length of the brain is under 10 cm (3.9 in), its volume measuring approximately 230 ml (8.1 imp fl oz; 7.8 US fl oz).[60][63][44]
The form of the brain is naturally narrow and elongated.[60][12] The cerebellum and cerebrum are both at high positions compared to modern ungulates that have brain hemispheres located above the cerebellum. Palmer noticed that the brain was similar to the modern aardvark (Orycteropus afer). The highly-developed cerebrum that enables a strong sense of smell from Anoplotherium makes it macrosmatic (derived in sense of smell), as also indicated by the enlarged olfactory bulbs and the small size of the neocortex.[60] In both Anoplotherium and Diplobune, the rhinal fissure divides the brain hemisphere horizontally and equally in half. The cerebellar vermis of the cerebellum is divided almost equally by the primary fissure of cerebellum (or "fissura prima").[64]
Additionally, the olfactory bulbs are thick, and the olfactory tubercles take the form of smooth circular elevations that are curved more backwards than the aardvark and are easily noticeable.[60] In another endocast for Anoplotherium, the olfactory bulbs compose 7.5% of the total volume of the brain, above average for both extinct and extant artiodactyls.[12]
The neocortex area of the brain, responsible for sensory perception and other sensory brain functions, covers 28% of the medium-sized A. commune endocast's surface area.[63] Another endocast, which belongs to Anoplotherium sp., measures 7,173.92 mm (282.438 in)2 in the cerebrum surface, 4,419.56 mm (173.998 in)2 in neopallium surface, and 416.09 cm (163.81 in)3 in endocranial volume. The former two data when calculated together (neopallium surface/cerebrum surface) compose 61.6% in the total neocortical surface area of the brain, meaning that adult Anoplotherium has massive brain and neocortical surface area measurements compared to most Paleogene artiodactyls, the latter measurement being on par with or less than those of modern artiodactyls.[12]
Anoplotherium and other anoplotheriids share traits of generally elongated and parallel sulci (shallow furrows) in the cerebral cortex, as well as a vertical (cordial) sulcus corresponding to the lateral (side) sulcus. The fissures (deep furrows) on the surface of the central area of the brain show clear formations of a complex lateral sulcus (also known as the Sylvian fissure) in a process known as operculization.[12] The operculization of the brain of anoplotheriids is similar to the Anthracotheriidae but does not indicate any close phylogenetic relation, which means that the similarities are an instance of parallel evolution. The measurements of the endocasts of Anoplotherium are larger than those of other Paleogene artiodactyls in a 2015 study by Ghislain Thiery and Stéphane Ducrocq.[65]
Dentition

Unlike most mammal fossil genera, Anoplotherium is diagnosed mainly based on postcranial morphology than dental morphology, but it does have diagnoses based on the latter.[44] The dental formula of Anoplotherium and other anoplotheriids is 3.1.4.33.1.4.3 for a total of 44 teeth, consistent with the primitive dental formula for early-middle Paleogene placental mammals.[57][66] Anoplotheriids have selenodont or bunoselenodont premolars and molars made for folivorous/browsing diets. The canines of the Anoplotheriidae are premolariform in shape, meaning that the canines are overall undifferentiated from other teeth like incisors. The lower premolars of the family are piercing and elongated. The upper molars are bunoselenodont in form while the lower molars have selenodont labial cuspids and bunodont lingual cuspids. The subfamily Anoplotheriinae, of which Anoplotherium is the type genus, differs from the Dacrytheriinae by the lower molars lacking a third cusp between the metaconid and entoconid as well as molariform premolars with crescent-shaped paraconules.[44]
The upper molars of Anoplotherium are characterized by trapezoidal outlines in occlusal views (or top views of the tooth enamel), W-shaped ectolophs (crests or ridges of upper molar teeth), and specific differences in cusps. More specifically, the upper molars of the genus contain near-central and conical protocone cusps closely aligned with the mesostyle cusps, conical paraconules that are connected to the parastyle by posterior crests, and compressed parastyles and mesostyles. The lower molars of the anoplotheriid contains the paraconid and metaconid cusps which have pronounced separations by a valley between them.[57][44]
Vertebrae and ribs

Anoplotherium has 7 total cervical vertebrae for a series of C1-C7, typical of most mammals. The atlas (C1) is similar to those of camelids such as Lama in form as well as the position of the "alar foramina" in association with facet joint connections involving the axis (C2).[11][23] An axis that was attributed to A. commune (but also possibly belonging to its close relative Diplobune secundaria) is elongated in length and has a diminished spinous process. The vertebrae C3-C7 are analogous to Cainotherium. The C4 vertebra appears slanted, which hints towards the neck changing in orientation from vertebra C3 to C4 as a potential bending in the front area of the neck, similar to modern bears. As a result of the neck vertebrae morphology, Anoplotherium likely had a sloped, upward position of the neck.[23]
Anoplotherium also had 12 thoracic vertebrae, 6 lumbar vertebrae, and 3 sacral vertebrae. The lumbar vertebrae, especially L4-L6, contain transverse processes that are wide, long, and point slightly towards a forward direction. The 3 sacral vertebrae are robust and contain apophyses for strong attachments to the long tail. The vertebrae of the anoplotheriid genus are built for typical ungulate movement.[11][23]
The most unusual postcranial aspect of Anoplotherium compared to other artiodactyls is the long and thick tail, which is made up of 22 caudal vertebrae for strong muscle support. The frontal vertebrae had well-pronounced process, and all vertebrae except for the farthest distal ones have haemal arches on them.[23]
Like the chalicothere Chalicotherium and unlike other mammals like caprines of the genus Ovis and Cainotherium, the ribs curve in wider areas and their tubercles do not project as much in the dorsal direction. The ribs of Anoplotherium form a barrel-shaped trunk, meaning that the rib cage is much wider than those of modern ruminants. The ribs generally project sideways due to the very curved positions of them, the position of the tubercle, and the thoracic vertebrae projecting on the upper sides.[23]
Limbs

Anoplotherium has short limbs and is thought to have been unguligrade in limb positions, with most species having three toes on both their front and hind limbs. A. commune is differentiated from the similar A. latipes by its didactyl ("toed-toed") as opposed to tridactyl ("three-toed") digits.[23][44][67]
Front limbs
The scapula (or shoulder blade) has a convex coracoid border and is similar to that of Diplobune. Similar to camels (Camelus), the supraspinous fossa is broader than the infraspinous fossa, but camels have narrower scapulae, especially in distal (back) ends of the supraspinous fossa. The scapular spine is robust, thick, and gradually rises in height distally up until it reaches the edge of the glenoid cavities like camels but unlike most other modern artiodactyls. The coracoid process (normally resembling a small hooklike structure) is reduced to a blunt knob that only slightly projects. The wide supraspinous fossa and broadly curved coracoid edge of the scapulae of Anoplotherium are unlike Cainotherium and Merycoidodon because Anoplotherium shares neither any triangular shape of the shoulder blades nor narrow supraspinous fossae.[23]
The elbow morphology of Anoplotherium, based on the structures and articulations of elbow bones like the humerus, radius, and ulna, shows evidence of adaptations to moving the elbow up and down in supination-pronation rotations by 13° maximum. A fully extended elbow could make an angle between the ulna and humerus that measures approximately 135°, indicating high flexibility compared to other artiodactyls, including the already semi-flexible elbows of Cainotherium.[23]
Similar in wrist morphology to pigs of the genus Sus, the hooves of Anoplotherium spread out by ~16° when downward, supported by footprint morphology. The wrist may have been able to rotate up and down but only to a limited degree and nowhere near the flexible wrist morphologies of primates, suggesting that the adaptation was not a main feature of the artiodactyl genus but the result of regaining a primitive trait.[23]
The carpus consists of the scaphoid, lunate, triquetrum, and pisiform in its first row and the trapezium, trapezoid, capitate, and hamate in its second. Anoplotherium has four digit bones, but those of digit V and, in the case of A. commune, digit II are poorly developed.[68] The second finger (digit II) of Anoplotherium has no capability of rotation or flexible movements, which signifies that it does not play any thumb-like role like in primates or the giant panda.[23]
Hind limbs

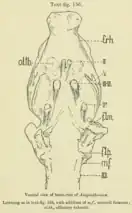
The ilium, part of the hip bone of the greater pelvis bone, is broad and has a firmly rounded iliac crest that meets with the concave underside edge at a sharp angle. The ilium of Anoplotherium can be differentiated from Palaeotherium by the shorter iliac body, the longer ischium (the lower and back area of the hip bone), and a straighter back edge of pelvis that results in a longer pubic symphysis. The acetabular fossa region of the acetabulum surface of the pelvis is large, its acetabular notch being in a posterior position similar to Chalicotherium.[23]
The femur is larger than the tibia, has only two trochanters similar to other basal artiodactyls, has a narrow gap between its femoral head and greater trochanter, and has a long femoral neck. The trochanteric fossa, a hollow at the surface of the greater trochanter, is wide in depth and narrow in shape, deepening by the sides. The tibia is robust, strongly supporting muscle attachments based on its crests and processes. The distal end of the fibula plus the medial malleolus prominence of the tibia enclose the center area of the astragalus in order to prevent it from moving sideways.[23]
Anoplotheriids with known postcranial fossils have proportionally wide, stocky, and oblique astragali (or talus or ankle bone), differing widely from other artiodactyls. A. latipes differs from A. commune in part by morphologies of the facets plus fossae of the astragalus and a shorter and more robust calcaneum (heel bone).[23][51] The astragali of anoplotheres share levels of elevations and positions of specific facets with the merycoidodonts that no modern artiodactyls share, possibly an instance of convergent evolution.[69][70] The medial (sustentacular) facet of Anoplotherium and Diplobune is concave, contrasting with the flat to slightly convex facet of Dacrytherium.
The tarsus consists of the navicular, three cueniform bones, and a cuboid bone. The foot of A. commune consisted of two toes, as indicated by the relatively small outermost and middle cuneiform bones.[68]
Footprints
Large-sized footprints from southern France and north Spain that date to the late Eocene[71] may have been from Anoplotherium. The ichnogenus is named Anoplotheriipus and was first described from the department of Gard in France by Paul Ellenberger in 1980. The derivation of the genus name refers to the ichnotaxon being closest in affinity to the Anoplotheriidae. The ichnogenus is diagnosed as belonging to a very large artiodactyl, the autopod area exceeding that of A. commune by ~33%, the subparallel position of the two hooves, and the posterior area of the pedal sole being as transversely wide as the anterior area of the pedal sole.[72] Anoplotheriipus is round to rectangular in shape with broad and anteriorly-pronounced cloven digit imprints that resemble poorly-preserved camel tracks.[73] The similar artiodactyl ichnogenus Diplartiopus differs from it by the parallelism of the two fingers that are more elongated.[74]
The type species is Anoplotheriipus lavocati, which Ellenberger named in honor of palaeontologist René Lavocat and considered the "most majestic" of the three ichnospecies due to the displayed specific mobility of the metatarsals. It measures 170 mm (6.7 in) to 180 mm (7.1 in) in length and 120 mm (4.7 in) in width, is stocky in shape, and measures 12° in toe divergence. The two fingers are nearly equal in length and, at minimum, measure 115 mm (4.5 in) without the metatarsal bones being taken into account and 225 mm (8.9 in) with the metatarsals. The measurements are considerably higher than typical measurements of the toes of A. commune, which are 85 mm (3.3 in) without the metatarsals and 170 mm (6.7 in) with.[72]
Anoplotheriipus similicommunis, deriving in species etymology from "similis" (similar in Latin) and A. commune, is similar to the type ichnospecies but is smaller, corresponding more directly to typical foot measurements of A. commune by its length of 140 mm (5.5 in) and width of 105 mm (4.1 in). The angle of divergence between the two main toes is 10°, and the minimum lengths of the fingers are 90 mm (3.5 in) without the metatarsals and 180 mm (7.1 in) with.[72]
Anoplotheriipus compactus is the third ichnospecies, which in species etymology derives from the Latin word "compactus" meaning "compact" in English due to the short and rounded autopod. It has a less definitive diagnosis compared to the other two ichnotaxa but is similar in size to A. similicommunis and has a nearly circular pedal sole for supporting slightly shorter fingers. Its length is 120 mm (4.7 in) while the width is 100 mm (3.9 in), and the finger lengths measure 70 mm (2.8 in) - 80 mm (3.1 in) without the metatarsals and 140 mm (5.5 in) - 150 mm (5.9 in) with. The footprints may have been produced by A. latipes although the answer is still uncertain.[72]
Size
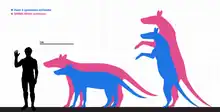
Anoplotherium species were particularly large in the late Eocene, reaching sizes unusual for most artiodactyl groups in the Paleogene. The large size estimates began in 1995 when Martinez and Sudre made weight estimates of Paleogene artiodactyls based on the dimensions of their astragali and M1 teeth. The astragali are common bones in fossil assemblages due to their reduced vulnerability to fragmentation as a result of their stocky shape and compact structure, explaining their choice for using it. The two measurements for A. commune yielded different results, with the M1 giving the body mass of 312.075 kg (688.01 lb) and the astragalus yielding 265.967 kg (586.36 lb). These estimates are far larger than those of most other Paleogene artiodactyls in the study, although the researchers pointed out that the M1 measurements could be overestimated compared to the astragalus estimate.[51]
In 2014, Takehisa Tsubamoto reexamined the relationship between astragalus size and estimated body mass based on extensive studies of extant terrestrial mammals, reapplying the methods to Paleogene artiodactyls previously tested by Sudre and Martinez. The researcher used linear measurements and their products with adjusted correction factors. The recalculations resulted in somewhat lower estimates compared to the 1995 results (with the exception of Diplobune minor, which as a shorter astragalus proportion than most other artiodactyls), displayed in the below graph:[75]
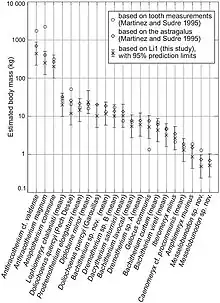
In 2022, Weppe calculated the body mass of A. commune, yielding 360 kg (790 lb).[52] In 2023, Ainara Badiola et. al. estimated that the weight of Anoplotherium ranges between 115 kg (254 lb) and 271 kg (597 lb). In their calculations, A. laurillardi was the smaller anoplotheriid that weighed on average 157 kg (346 lb). A. latipes was larger and has an average weight estimate of 229 kg (505 lb), and A. commune has the heaviest weight estimates at 271 kg (597 lb).[44]
In 2007, Hooker made size estimates of A. latipes based on an incomplete skeleton of an immature individual from the Hamstead Member of the Bouldnor Formation in the Isle of Wight, United Kingdom. The reconstructed Hamstead level 3 individual gave size measurements of 2 m (6 ft 7 in) in head and body length. The immature Anoplotherium individual's humerus measures 330 mm (13 in) long, so the humeri of mature individuals may have measured about 410 mm (16 in) long. As a result, adult A. latipes may have measured 2.5 m (8 ft 2 in) in head and body length and 1.25 m (4 ft 1 in) in shoulder height. When standing up bipedally on its hind limbs with the back, neck and head at an angle of about 15°, the Hamstead level 3 individual might have reached 2.5 m (8 ft 2 in) when browsing while more mature A. latipes individuals might have stood just over 3 m (9.8 ft).[23]
Palaeobiology

Since 2007, Anoplotherium is thought to have been a quadruped that could have stood on its hind legs as a bipedal browser thanks to the strong pelvis, long and robust tail for balance, and splayed hind legs. The bipedal adaptations show some instance of convergence with other animals like chalicotheres, various genera of ground sloths, giant pandas (Ailuropoda melanoleuca), gorillas (Gorilla), and the gerenuk (Litocranius walleri). Otherwise, the general body form appears to resemble those of the Canidae. As a result of the bend C3-C4 cervical vertebrae, the neck and head could have maintained horizontal orientations while standing bipedally. The forelimbs could have extended horizontally beyond the snout while the individual stood bipedally, although it could not have reached upward and did not have claws or prehensile organs on the manus unlike Chalicotherium. Therefore, the forearms may have not been used for ripping and tearing plants but as bipedal support. It may have browsed while standing up at a steep angle more comparable to the gerenuk than to Chalicotherium.[23]
Its large size and ability to bipedally browse may have given Anoplotherium few sources of terrestrial competition other than from Palaeotherium magnum, a large-sized palaeothere with a long neck that may have reached 240.3 kg (530 lb) in body mass.[23][76] The subspecies P. magnum magnum would have reached just over 2 m (6 ft 7 in) in browsing height in quadruped stance, and there is no evidence for any bipedal adaptation in palaeotheres.[23] Anoplotherium likely engaged in degrees of niche partitioning with the late Eocene palaeotheres and Diplobune. While all were folivorous browsers, the palaeotheres Plagiolophus and Palaeotherium may have had small degrees of frugivory while Diplobune was likely adapted to arborealism.[77][78][67] How well-adapted Anoplotherium was to abrasive leaves and drier but still subhumid conditions in the late Eocene is not well-known and requires future research in dentition for answers.[23]

Hooker proposed the possibility that the didactyl A. commune and A. latipes may have been sexual dimorphs of the same species (in which A. latipes would be a synonym of A. commune). There are little consistent differences in dental morphology between the two species, with any small differences potentially accounting for individual variations. The differences in toe number between the species may have reflected A. latipes being three-toed and A. commune being two-toed. The palaeontologist explained that while there is no evidence for the extra digit touching the ground while the individual was walking, the extra digit of A. latipes may have served as extra balance while browsing bipedally.[23]
The third digit might have also served as part of sparring in intraspecific competition between male individuals. However, he noted that despite the apparent "advantage" of A. latipes in bipedal browsing, there is no evidence of sexual differences in dietary behaviours or preferences. In addition, both species are found in the same localities of Bouldnor in the United Kingdom plus La Débruge and Montmartre in France, that although A. latipes is more common in La Débruge than Montmartre, this may be the results of behavioural and/or taphonomic factors.[23][44] Grégoire Métais expressed being unconvinced that the third toe of A. latipes is a sexually dimorphic adaptation for bipedal browsing, instead suggesting that they were used in male sparring if A. latipes and A. commune were sexual dimorphs.[67]
Some evidence of the morphologies of Anoplotherium have been criticized by some sources. In their study of the morphology of the gerenuk that allows for bipedal, researchers Matt Cartmill and Kaye Brown argued that several postcranial features that were supposedly adaptations of Litocranius and other bipedal genera does not distinguish the gerenuk from other bovids.[79] Ciaran Clark et. al. (including J.J. Hooker) found from micro-CT scans that Anoplotherium being a facultative bipedal browser was not supported by the trabecular architecture of the proximal area of the femur. This may have been the result of poor data results from the micro-CT scans and the smaller sample size, which higher-contrast micro-CT data may better answer in postural information.[80]
The footprint track patterns of Anoplotheriipus suggest that Anoplotherium walked in very similar movement speeds as each other. Based on groupings of the footprint ichnotaxon within the locality of Fondota in the municipality of Abiego in Spain, Anoplotherium may have commonly walked in small groups which may imply some gregarious (or sociable) behaviour.[81]
Palaeoecology
Early pre–Grande Coupure Europe
For much of the Eocene, a hothouse climate with humid, tropical environments with consistently high precipitations prevailed. Modern mammalian orders including the Perissodactyla, Artiodactyla, and Primates (or the suborder Euprimates) appeared already by the early Eocene, diversifying rapidly and developing dentitions specialized for folivory. The omnivorous forms mostly either switched to folivorous diets or went extinct by the middle Eocene (47 to 37 Ma) along with the archaic "condylarths". By the late Eocene (approximately 37 to 33 Ma), most of the ungulate form dentitions shifted from bunodont cusps to cutting ridges (i.e. lophs) for folivorous diets.[82][83]
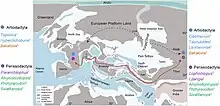
Land-based connections to the north of the developing Atlantic Ocean were interrupted around 53 Ma, meaning that North America and Greenland were no longer well-connected to western Europe. From the early Eocene up until the Grande Coupure extinction event (56 Ma to 33.9 Ma), the western Eurasian continent was separated into three landmasses, the former two of which were isolated by seaways: western Europe (an archipelago), Balkanatolia, and eastern Eurasia (Balkanatolia was in between the Paratethys Sea of the north and the Neotethys Ocean of the south).[46] The Holarctic mammalian faunas of western Europe were therefore mostly isolated from other continents including Greenland, Africa, and eastern Eurasia, allowing for endemism to occur within western Europe.[83] The European mammals of the late Eocene (MP17 to MP20) were mostly descendants of endemic middle Eocene groups as a result.[84]
The appearances of derived anoplotheriines by MP18 occurred long after the extinction of the endemic European perissodactyl family Lophiodontidae in MP16, including the largest lophiodont Lophiodon lautricense, likely the result of a shift from humid and highly tropical environments to drier and more temperate forests with open areas and more abrasive vegetation. The surviving herbivorous faunas shifted their dentitions and dietary strategies accordingly to adapt.[85][86] The environments were still subhumid and full of subtropical evergreen forests, however. The Palaeotheriidae was the sole remaining European perissodactyl group, and frugivorous-folivorous or purely folivorous artiodactyls became the dominant group in western Europe.[87][88] MP16 also marked the last appearances of most European crocodylomorphs, of which the aligatoroid Diplocynodon was the only survivor due to seemingly adapting to the general decline of tropical climates of the late Eocene.[89][90][91]
Late Eocene
After a considerable gap in anoplotheriine fossils in MP17a and MP17b, the derived anoplotheriines Anoplotherium and Diplobune made their first known appearances in the MP18 unit.[49] They were exclusive to the western European archipelago, but their exact origins and dispersal routes are unknown. By then, Anoplotherium and Diplobune lived in Central Europe (then an island) and the Iberian Penninsula, only the former genus of which later dispersed into southern England by MP19 due to the apparent lack of ocean barriers.[44][23]
Anoplotherium coexisted with a wide diversity of artiodactyls in western Europe by MP18, ranging from the more widespread Dichobunidae, Tapirulidae, and Anthracotheriidae to many other endemic families consisting of the Xiphodontidae, Choeropotamidae (recently determined to be polyphyletic, however), Cebochoeridae, Amphimerycidae, Mixtotheriidae, and Cainotheriidae.[47][53][92][93] Anoplotherium also coexisted with the Palaeotheriidae, the remaining perissodactyl family of western Europe.[84] Late Eocene European groups of the clade Ferae represented predominantly the Hyaenodonta (Hyaenodontinae, Hyainailourinae, and Proviverrinae) but also contained Carnivoramorpha (Miacidae) and Carnivora (small-sized Amphicyonidae).[87] Other mammal groups present in the late Eocene of western Europe represented the leptictidans (Pseudorhyncocyonidae),[94] primates (Adapoidea and Omomyoidea),[95] eulipotyphlans (Nyctitheriidae),[96] chiropterans,[83] herpetotheriid marsupials,[97], apatotherians,[98] and endemic rodents (Pseudosciuridae, Theridomyidae, and Gliridae).[99] The alligatoroid Diplocynodon, present only in Europe since the upper Paleocene, coexisted with pre-Grande Coupure faunas as well.[100] In addition to snakes, frogs, and salamandrids, rich assemblage of lizards are known in western Europe as well from MP16-MP20, representing the Iguanidae, Lacertidae, Gekkonidae, Agamidae, Scincidae, Helodermatidae, and Varanoidea.[101]
In the MP18 locality of Zambrana in Spain, A. laurillardi and A. sp. remains were found with undetermined frog and squamate groups, alligatoroid Diplocynodon, the herpetotheriid Peratherium, rodents (Theridomys, Elfomys, Pseudoltinomys, Remys), omomyid Microchoerus, carnivoraformes Quercygale and Paramiacis, dichobunid Dichobune, xiphodonts Xiphodon and Haplomeryx, and palaeotheres (Palaeotherium, Leptolophus, Iberolophus, Pachynolophus, Paranchilophus).[102]
As part of a separate landmass at the time, La Débruge of France, dating to MP18, yielded slightly different faunas that coexisted with A. commune, A. latipes, and A. laurillardi, namely the herpetotheriid Peratherium, rodents (Blainvillimys, Theridomys, Plesiarctomys, Glamys), hyaenodonts (Hyaenodon and Pterodon), amphicyonid Cynodictis, palaeotheres (Plagiolophus, Anchilophus, Palaeotherium), dichobunid Dichobune, choeropotamid Choeropotamus, cebochoerids Cebochoerus and Acotherulum, anoplotheriids Dacrytherium and Diplobune, tapirulid Tapirulus, xiphodonts Xiphodon and Dichodon, cainothere Oxacron, amphimerycid Amphimeryx, and anthracothere Elomeryx.[103]
Extinction

The Grande Coupure extinction and faunal turnover event of western Europe, dating back to the earliest Oligocene (MP20-MP21), is one of the largest and most abrupt faunal events in the Cenozoic record, which is coincident with climate forcing events of cooler and more seasonal climates.[104] The result of the event was a 60% extinction rate of western European mammalian lineages while Asian faunal immigrants replaced them.[105][106][107] The Grande Coupure is often marked by palaeontologists as part of the Eocene-Oligocene boundary as a result at 33.9 Ma, although some estimate that the event began 33.6-33.4 Ma.[108][109] The event correlates directly with or after the Eocene-Oligocene transition, an abrupt shift from a greenhouse world characterizing much of the Paleogene to a coolhouse/icehouse world of the early Oligocene onwards. The massive drop in temperatures stems from the first major expansion of the Antarctic ice sheets that caused drastic pCO2 decreases and an estimated drop of ~70 m (230 ft) in sea level.[110]
The seaway dynamics separating western Europe from other landmasses to strong extents but allowing for some levels of dispersals prior to the Grande Coupure are complicated and contentious, but many palaeontologists agreed that glaciation and the resulting drops in sea level played major roles in the drying of the seaways previously acting as major barriers to eastern migrants from Balkanatolia and western Europe. The Turgai Strait is often proposed as the main European seaway barrier prior to the Grande Coupure, but some researchers challenged this perception recently, arguing that it completely receded already 37 Ma, long before the Eocene-Oligocene transition. Alexis Licht et. al suggested that the Grande Coupure could have possibly been synchronous with the Oi-1 glaciation (33.5 Ma), which records a decline in atmospheric CO2, boosting the Antarctic glaciation that already started by the Eocene-Oligocene transition.[46][111]
The Grande Coupure event also marked a large faunal turnover marking the arrivals of later anthracotheres, entelodonts, ruminants (Gelocidae, Lophiomerycidae), rhinocerotoids (Rhinocerotidae, Amynodontidae, Eggysodontidae), carnivorans (later Amphicyonidae, Amphicynodontidae, Nimravidae, and Ursidae), eastern Eurasian rodents (Eomyidae, Cricetidae, and Castoridae), and eulipotyphlans (Erinaceidae).[112][113][105][114]
The Eocene-Oligocene transition of western Europe, as a result of the global climatic conditions, is marked by a transition from tropical and subtropical forests to more open, temperate or mixed deciduous habitats with adaptations to increased seasonality. While Anoplotherium did not last long in the earliest Oligocene, there are disagreements as to whether it survived the Grande Coupure or went extinct at the event.[88][47] While evidence points towards Anoplotherium being extirpated from areas like France and the United Kingdom by the Grande Coupure (last occurrences MP20),[103][105][52] the perception is complicated by the apparent last survival of A. commune in the MP21 locality of Möhren 19 in southern Germany (the edge of western Europe) along with Palaeotherium medium and Diplobune quercyi (slightly younger localities indicate their extinctions and replacements by Grande Coupure immigrants such as the anthracothere Anthracotherium and the rhinocerotid Epiaceratherium).[115]
Hooker pointed out that localities like Möhren 19 span earlier times where the surviving endemic faunas are accompanied by some Grande Coupure immigrants but otherwise were not yet joined by certain immigrants such as Anthracotherium. Additionally, the surviving endemics of the locality are missing from other areas dating to MP21. Therefore, he argued that certain older MP21 localities with surviving endemic faunas fill the long gap between the youngest pre-Grande Coupure Lower Hamstead Member and the younger post-Grande Coupure Upper Hamstead Member within the Bouldnor Formation. This interpretation, Hooker explained, means that the localities represented very brief moments of survival of endemic faunas during the Grande Coupure, therefore supporting the idea of a major and rapid faunal extinction and immigration event, including the extinction of Anoplotherium in the event.[105][116]
The extinctions of a majority of endemic artiodactyls, including Anoplotherium, have been attributed to competition with immigrant faunas, environmental changes from cooling climates, or some combination of the two.[108] Sarah C. Joomun et. al. determined that certain faunas may have arrived later and therefore may have not played roles in the extinctions. They concluded that climate change, which led to increased seasonality and changes in plant food availability, led the artiodactyls to become unable to adapt to the major changes and go extinct.[117] Weppe made similar arguments towards climate change being the main cause of the Grande Coupure extinction event, arguing that the cooling climates displaced the previously stable subtropical environments of western Europe and caused a collapse in the artiodactyl community, which after their extinctions left empty ecological niches that were passively filled by immigrant faunas.[52]
Notes
- ↑ The French adjective commune translates in English to 'common'.
References
- ↑ Cuvier, Georges (1804). "Suite des Recherches: Sur les espèces d'animaux dont proviennent les os fossiles répandus dans la pierre à plâtre des environs de Paris". Annales du Muséum National d'Histoire Naturelle, Paris (in French). 3: 364–387. Archived from the original on 2023-07-27. Retrieved 2023-08-30.
- ↑ Rudwick, Martin J. S. (2022). "Georges Cuvier's appeal for international collaboration, 1800". History of Geology. 46 (1): 117–125. doi:10.18814/epiiugs/2022/022002. S2CID 246893918.
- ↑ Roberts, George (1839). An etymological and explanatory dictionary of the terms and language of geology. London: Longman, Orme, Brown, Green, & Longmans. p. 8. Retrieved 29 December 2021.
- ↑ Cuvier, Georges (1804). "Suite des Recherches: Suite de recherches sur les os fossiles de la pierre à plâtre des environs de Paris. Troisième mémoire. Restitution des pieds. Première section. Restitution des différens pieds de derrière". Annales du Muséum National d'Histoire Naturelle, Paris (in French). 3: 442–472. Archived from the original on 2023-07-27. Retrieved 2023-08-30.
- ↑ Cuvier, Georges (1805). "Troisième mémoire. Deuxième section. Restitution des différens pieds de devant". Annales du Muséum National d'Histoire Naturelle, Paris (in French). 6: 253–283. Archived from the original on 2012-11-10. Retrieved 2023-08-30.
- ↑ Cuvier, Georges (1807). "Suite des recherches sur les os fossiles des environs de Paris. Troisième mémoire, troisième section, les phalanges. Quatrième mémoire sur les os des extrémités, première section, les os longs des extrémités postérieures". Annales du Muséum d'Histoire Naturelle. 9: 10–44. Archived from the original on 2023-09-02. Retrieved 2023-08-30.
- 1 2 Cuvier, Georges (1807). "Suite des recherches sur les os fossiles des environs de Paris. Ve mémoire, IIe section, description de deux squelettes presque entiers d'Anoplotherium commune". Annales du Muséum d'Histoire Naturelle (in French). 9: 272–282. Archived from the original on 2023-09-02. Retrieved 2023-08-30.
- ↑ Belhoste, Bruno (2017). "Chapter 10: From Quarry to Paper. Cuvier's Three Epistemological Cultures". In Chemla, Karine; Keller, Evelyn Fox (eds.). Cultures without Culturalism: The Making of Scientific Knowledge. Duke University Press. pp. 250–277.
- ↑ Wallace, David Rains (2004). "Chapter 1: Pachyderms in the Catacombs". Beasts of Eden: Walking Whales, Dawn Horses, and Other Enigmas of Mammal Evolution. University of California Press. pp. 1–13.
- ↑ Allemand, Rémi (2017). Endocranial microtomographic study of marine reptiles (Plesiosauria and Mosasauroidea) from the Turonian (Late Cretaceous) of Morocco: palaeobiological and behavioral implications (PhD). National Museum of Natural History, France.
- 1 2 3 4 Cuvier, Georges (1822). Recherches sur les ossemens fossiles, où l'on rétablit les caractères de plusieurs animaux dont les révolutions du globe ont détruit les espèces. Vol. 3. G. Dufour and E. d'Ocagne. Archived from the original on 2023-08-19. Retrieved 2023-08-30.
- 1 2 3 4 5 6 Orliac, Maeva J.; Maugoust, Jacob; Balcarcel, Ana; Gilissen, Emmanuel (2023). "Paleoneurology of Artiodactyla, an Overview of the Evolution of the Artiodactyl Brain" (PDF). In Dozo; Paulina-Carabajal, Ariana; Macrini, Thomas E.; Walsh, Stig (eds.). Paleoneurology of Amniotes. Springer Cham. pp. 507–555. doi:10.1007/978-3-031-13983-3_13. ISBN 978-3-031-13982-6. Archived (PDF) from the original on 2023-08-29. Retrieved 2023-08-30.
- ↑ Moodie, Roy Lee (1917). "Studies in Paleopathology. I. General Consideration of Evidence of Pathological Conditions Found among Fossil Animals". Annals of Medical History. 1 (4): 374–393. PMC 7927727. PMID 33943144.
- ↑ Diéguez, Carmen; Isidro, Albert; Malgosa, Assumpció (1996). "An introduction to zoo-paleopathology and an update on fossil phyto-paleopathology from Spain". Journal of Paleopathology. 8 (3): 133–142.
- ↑ Thomas, Richard (2012). "Chapter 66: NonHuman Paleopathology". In Buikstra, Jane; Roberts, Charlotte (eds.). The Global History of Paleopathology: Pioneers and Prospects. Oxford University Press. pp. 652–664. doi:10.1093/acprof:osobl/9780195389807.003.0066.
- ↑ Cuvier, Geoges (1812). "Résumé général et rétablissement des Squelettes des diverses espèces". Recherches sur les ossemens fossiles de quadrupèdes: où l'on rétablit les caractères de plusieurs espèces d'animaux que les révolutions du globe paroissent avoir détruites (in French). Vol. 3. Chez Deterville. Archived from the original on 2023-07-31. Retrieved 2023-08-30.
- ↑ Rudwick, Martin J. S. (1997). "Chapter 6: The Animals from the Gypsum Beds around Paris". Georges Cuvier, Fossil Bones, and Geological Catastrophes: New Translations and Interpretations of the Primary Texts. University of Chicago Press.
- ↑ Manucci, Fabio; Romano, Marco (2022). "Reviewing the iconography and the central role of 'paleoart': four centuries of geo-palaeontological art". Historical Biology. 35 (1): 1–48. doi:10.1080/08912963.2021.2017919. S2CID 246054069.
- ↑ Rudwick, Martin J.S. (1992). "Chapter 2: Keyholes into the Past". Scenes from Deep Time: Early Pictorial Representations of the Prehistoric World. pp. 27–58.
- 1 2 Gervais, Paul (1848–1852). "Note sur le genre Eurytherium, suivie d'une liste comparative des Mamifères observés dans les hassins de Paris et d'Apt, et de remarques sur les Ongulés observés en France.". Zoologie et paléontologie françaises (animaux vertébrés): ou nouvelles recherches sur les animaux vivants et fossiles de la France. Vol. 2. Arthus Bertrand. Archived from the original on 2023-08-04. Retrieved 2023-08-30.
- 1 2 Schlosser, Max (1883). "Uebersicht der bekannten Anoplotherien und Diplobunen nebst Erläuterung der Beziehungen zwischen Anoplotherium und anderen Säugethierfamilien". Neues Jahrbuch für Mineralogie, Geologie und Palaeontologie, Abhandlungen. 2. Archived from the original on 2023-08-04. Retrieved 2023-08-30.
- ↑ Dor, M. (1938). "Sur la biologie de l'Anoplotherium (L'Anoplotherium était-il aquatique?)". Mammalia. 2: 43–48.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Hooker, Jerry J. (2007). "Bipedal browsing adaptations of the unusual Late Eocene–earliest Oligocene tylopod Anoplotherium (Artiodactyla, Mammalia)". Zoological Journal of the Linnean Society. 151 (3): 609–659. doi:10.1111/j.1096-3642.2007.00352.x.
- ↑ Davydenko, Svitozar; Gol’din, Pavel; Bosselaers, Mark; Vahldiek, Bernd; Vliet, Henk Jan van (2023). "Gross and microscopic anatomy of a tibia tentatively attributed to a cetacean from the Middle Eocene of Europe, with a note on the artiodactyl Anoplotherium and on the perissodactyl Lophiodon". Paläontologische Zeitschrift. 97 (3): 627–652. Bibcode:2023PalZ...97..627D. doi:10.1007/s12542-023-00653-x. S2CID 259897461.
- ↑ Phillips, Samuel; Shenton, Francis Kingston John (1860). Guide to the Crystal Palace and park. Archived from the original on 2023-08-29. Retrieved 2023-08-30.
- 1 2 Witton, Mark P.; Michel, Ellinor (2022). "Chapter 4: The sculptures: mammals". The Art and Science of the Crystal Palace Dinosaurs. The Crowood Press. pp. 68–91.
- ↑ Gervais, Paul (1848–1852). "Diverses espèces d'Ongulés fossiles.". Zoologie et paléontologie françaises (animaux vertébrés): ou nouvelles recherches sur les animaux vivants et fossiles de la France. Vol. 2. Arthus Bertrand. Archived from the original on 2023-08-04. Retrieved 2023-08-30.
- 1 2 3 4 Lydekker, Richard (1885). Catalogue of the fossil Mammalia in the British museum, (Natural History): Part II. Containing the Order Ungulata, Suborder Artiodactyla. Order of the Trustees, London. Archived from the original on 2023-08-02. Retrieved 2023-08-30.
- ↑ Saint-Hilaire, Étienne Geoffroy (1833). "Considérations sur ossements fossiles, la plus inconnus, trouvés et observés dans le l'Auvergne". Revue encyclopédique, ou Analyse raisonnée des productions les plus remarquables. Vol. 59. Bureau Central de la Revue Encyclopédique. Archived from the original on 2023-08-25. Retrieved 2023-09-19.
- 1 2 Pomel, Auguste (1851). "Nouvelles observations sur la structure des pieds dans les animaux de la famille des Anoplotherium, et dans le genre Hyaemoschus". Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences. 33: 16–17. Archived from the original on 2023-08-03. Retrieved 2023-08-30.
- ↑ Berthet, Didier (2003). "Le genre Cainotherium (Mammalia, Artiodactyla): étude morphométrique, révision systématique, implications évolutives et paléogéographiques, extinction". Travaux et Documents des Laboratoires de Géologie de Lyon. 159 (159): 3–205. Archived from the original on 2023-08-06. Retrieved 2023-08-30.
- ↑ Lartet, Édouard (1837). "Note sur les ossements fossiles des terrains tertiaires de Simorre, de Sansan, etc., dans le département du Gers, et sur la découverte récente d'une mâchoire de singe fossile". Comptes Rendus de l'Académie des Sciences. 4: 85–93. Archived from the original on 2020-06-13. Retrieved 2023-08-30.
- ↑ Lartet, Édouard (1839). "Notice géologique". Extrait de l'Annuaire du Département du Gers.
- ↑ Anquetin, Jérémy; Antoine, Pierre-Olivier; Tassy, Pascal (2007). "Middle Miocene Chalicotheriinae (Mammalia, Perissodactyla) from France, with a discussion on chalicotheriine phylogeny". Zoological Journal of the Linnean Society. 151 (3): 577–608. doi:10.1111/j.1096-3642.2007.00327.x.
- ↑ Cautley, Proby T.; Falconer, Hugh (1837). "Notice on the Remains of a Fossil Monkey from the Tertiary Strata of the Sewalik Hills in the North of Hindoostan". Transactions of the Geological Society: 499–504. Archived from the original on 2023-08-02. Retrieved 2023-08-30.
- ↑ Lydekker, Richard (1886). Catalogue of the fossil Mammalia in the British museum, (Natural History): Part III. Containing the Order Ungulata, Suborders Perissodactyla, Toxondontia, Condylarthra, and Amblypoda. Order of the Trustees, London. Archived from the original on 2023-08-02. Retrieved 2023-08-30.
- ↑ Colbert, Edwin H. (1935). "The Proper Use of the Generic Name Nestoritherium". Journal of Mammalogy. 16 (3): 233–234. doi:10.1093/jmammal/16.3.233.
- ↑ Buffetaut, Eric (2016). "From Charles Darwin's comments to the first mention of South American giant fossil birds: Auguste Bravard's catalogue of fossil species from Argentina (1860) and its significance". Bulletin de la Société Géologique de France. 187 (1): 41–53. doi:10.2113/gssgfbull.187.1.41.
- ↑ Filhol, Henri (1877). "Recherches sur les Phosphorites du Quercy. Etude des fossiles qu'on y rencontre et spécialement des mammiféres". Annales des Sciences Géologiques de Paris. Archived from the original on 2023-08-04. Retrieved 2023-08-30.
- ↑ Rütimeyer, Ludwig (1862). "Eocaene Säugethiere aus dem Gebiet des schweizerischen Jura". Neue Denkschriften der Schweizerischen Naturforschenden Gesellschaft. 19: 1–98. Archived from the original on 2023-08-04. Retrieved 2023-08-30.
- ↑ von Fraas, Oscar Friedrich (1870). "Diplobune bavaricum". Palaeontographica. 17: 177–184. Archived from the original on 2023-08-04. Retrieved 2023-08-30.
- ↑ Stehlin, Hans Georg (1910). "Die Säugertiere des schweizerischen Eocaens. Sechster Teil: Catodontherium – Dacrytherium – Leptotherium – Anoplotherium – Diplobune – Xiphodon – Pseudamphimeryx – Amphimeryx – Dichodon – Haplomeryx – Tapirulus – Gelocus. Nachträge, Artiodactyla incertae sedis, Schlussbetrachtungen über die Artiodactylen, Nachträge zu den Perissodactylen". Abhandlungen der Schweizerischen Paläontologischen Gesellschaft. 36. Archived from the original on 2023-08-05. Retrieved 2023-08-30.
- ↑ Dietrich, Wilhelm Otto (1922). "Beitrag zur Kenntnis der säugetierführenden Bohnerzformation in Schwaben. 1. Ein vergessenes, neu erschlossenes Höhlenvorkommen terrestrischen Eozäns auf der Ulmer Alb". Zentralblatt für Mineralogie, Geologie und Paläontologie. 19: 209–224.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Badiola, Ainara; De Vicuña, Nahia Jiménez; Perales-Gogenola, Leire; Gómez-Olivencia, Asier (2023). "First clear evidence of Anoplotherium (Mammalia, Artiodactyla) in the Iberian Peninsula: an update on the Iberian anoplotheriines". The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. doi:10.1002/ar.25238. PMID 37221992. S2CID 258864256.
- ↑ de Bonis, Louis (1964). "Étude de quelques mammifères du Ludien de La Débruge (Vaucluse)". Annales de Paléontologie: 121–154.
- 1 2 3 Licht, Alexis; Métais, Grégoire; Coster, Pauline; İbilioğlu, Deniz; Ocakoğlu, Faruk; Westerweel, Jan; Mueller, Megan; Campbell, Clay; Mattingly, Spencer; Wood, Melissa C.; Beard, K. Christopher (2022). "Balkanatolia: The insular mammalian biogeographic province that partly paved the way to the Grande Coupure". Earth-Science Reviews. 226: 103929. Bibcode:2022ESRv..22603929L. doi:10.1016/j.earscirev.2022.103929.
- 1 2 3 4 Erfurt, Jörg; Métais, Grégoire (2007). "Endemic European Paleogene Artiodactyls". In Prothero, Donald R.; Foss, Scott E. (eds.). The Evolution of Artiodactyls. Johns Hopkins University Press. pp. 59–84.
- ↑ Orliac, Maeva; Gilissen, Emmanuel (2012). "Virtual endocranial cast of earliest Eocene Diacodexis (Artiodactyla, Mammalia) and morphological diversity of early artiodactyl brains". Proceedings of the Royal Society B. 279 (1743): 3670–3677. doi:10.1098/rspb.2012.1156. PMC 3415922. PMID 22764165.
- 1 2 3 4 Cuesta, Miguel-Ángel; Badiola, Ainara (2009). "Duerotherium sudrei gen. et sp. nov., a New Anoplotheriine Artiodactyl from the Middle Eocene of the Iberian Peninsula". Journal of Vertebrate Paleontology. 29 (1): 303–308. Bibcode:2009JVPal..29..303C. doi:10.1671/039.029.0110. JSTOR 20491092. S2CID 55546022. Archived from the original on 2023-08-10. Retrieved 2023-08-30.
- ↑ Schmidt-Kittler, Norbert; Godinot, Marc; Franzen, Jens L.; Hooker, Jeremy J. (1987). "European reference levels and correlation tables". Münchner geowissenschaftliche Abhandlungen A10. Pfeil Verlag, München. pp. 13–31.
- 1 2 3 Sudre, Jean; Martinez, Jean-Noël (1995). "The astragalus of Paleogene artiodactyls: comparative morphology, variability and prediction of body mass". Lethaia. 28 (3): 197–209. Bibcode:1995Letha..28..197M. doi:10.1111/j.1502-3931.1995.tb01423.x.
- 1 2 3 4 5 6 Weppe, Romain (2022). Déclin des artiodactyles endémiques européens, autopsie d'une extinction (Thesis) (in French). University of Montpellier. Archived from the original on 2023-08-11. Retrieved 2023-08-30.
- 1 2 Luccisano, Vincent; Sudre, Jean; Lihoreau, Fabrice (2020). "Revision of the Eocene artiodactyls (Mammalia, Placentalia) from Aumelas and Saint-Martin-de-Londres (Montpellier limestones, Hérault, France) questions the early European artiodactyl radiation". Journal of Systematic Palaeontology. 18 (19): 1631–1656. Bibcode:2020JSPal..18.1631L. doi:10.1080/14772019.2020.1799253. S2CID 221468663.
- 1 2 Weppe, Romain; Blondel, Cécile; Vianey-Liaud, Monique; Escarguel, Gilles; Pélissié, Thierry; Antoine, Pierre-Olivier; Orliac, Maëva Judith (2020). "Cainotheriidae (Mammalia, Artiodactyla) from Dams (Quercy, SW France): phylogenetic relationships and evolution around the Eocene–Oligocene transition (MP19–MP21)" (PDF). Journal of Systematic Palaeontology. 18 (7): 541–572. Bibcode:2020JSPal..18..541W. doi:10.1080/14772019.2019.1645754. S2CID 202026238. Archived (PDF) from the original on 2022-03-07. Retrieved 2023-09-19.
- ↑ Weppe, Romain; Blondel, Cécile; Vianey-Liaud, Monique; Pélissié, Thierry; Orliac, Maëva Judith (2020). "A new Cainotherioidea (Mammalia, Artiodactyla) from Palembert (Quercy, SW France): Phylogenetic relationships and evolutionary history of the dental pattern of Cainotheriidae". Palaeontologia Electronica (23(3):a54). doi:10.26879/1081. S2CID 229490410.
- ↑ Gentry, Alan W.; Hooker, Jerry J. (1988). "The phylogeny of the Artiodactyla". The Phylogeny and Classification of the Tetrapods: Volume 2: Mammals (The Systematics Association Special Volume, No. 35B). Oxford University Press. pp. 235–272.
- 1 2 3 von Zittel, Karl Alfred (1925). Schlosser, Max (ed.). Text-Book of Paleontology. Volume III. Mammalia. Macmillan and Co. Limited. pp. 179–180. Archived from the original on 2023-08-14. Retrieved 2023-08-30.
- ↑ Beddard, Frank Evers (1902). Harmer, Sidney Frederic; Shipley, Arthur Everett (eds.). The Cambridge Natural History: Mammalia. Macmillan and Co. Limited. pp. 332–333. Archived from the original on 2023-08-17. Retrieved 2023-08-30.
- ↑ Scott, William B. (1945). "The Mammalia of the Duchesne River Oligocene". Transactions of the American Philosophical Society. 34 (3): 209–253. doi:10.2307/1005542. JSTOR 1005542.
- 1 2 3 4 5 6 7 Palmer, R.W. (1913). "The Brain and Brain-Case of a Fossil Ungulate of the Genus Anoplotherium". Proceedings of the Zoological Society of London. 83 (4): 878–893. doi:10.1111/j.1096-3642.1913.tb01994.x. Archived from the original on 2023-10-13. Retrieved 2023-09-19.
- ↑ Pearson, Helga Sharpe (1927). "On the Skulls of Early Tertiary Suidae, together with an Account of the Otic Region in Some Other Primitive Artiodactyla". Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character. 215 (421–430): 440–445. doi:10.1098/rstb.1927.0009.
- ↑ Gagnaison, Cyril; Leroux, Jean-Jacques (2013). "Un crâne de Diplobune secundaria Cuvier, 1822 de Saint-Capraise-d'Eymet (Dordogne)". Symbioses (in French). 29: 43–46.
- 1 2 Jerison, Harry J. (2009). "Chapter 10: How Can Fossils Tell us About the Evolution of the Neocortex?". In Kass, Jon H.; Striedter, Georg F.; Rubenstein, John L.R.; Bullock, Theodore H.; Krubitzer, Leah; Preuss, Todd (eds.). Evolutionary Neuroscience. Academic Press. pp. 497–508.
- ↑ Edinger, Tilly (1948). "Evolution of the Horse Brain". 25 : Evolution of the Horse Brain. Geological Society of America Memoirs. Vol. 25. Geological Society of America. pp. 1–178. doi:10.1130/MEM25-p1. Archived from the original on 2023-10-13. Retrieved 2023-09-19.
- ↑ Thiery, Ghislain; Ducrocq, Stéphane (2015). "Endocasts and brain evolution in Anthracotheriidae (Artiodactyla, Hippopotamoidea)". Journal of Anatomy. 227 (3): 277–285. doi:10.1111/joa.12348. PMC 4560562. PMID 26278931.
- ↑ Lihoreau, Fabrice; Boisserie, Jean-Renaud; Viriot, Laurent; Brunet, Michel (2006). "Anthracothere dental anatomy reveals a late Miocene Chado-Libyan bioprovince". Proceedings of the National Academy of Sciences. 103 (23): 8763–8767. Bibcode:2006PNAS..103.8763L. doi:10.1073/pnas.0603126103. PMC 1482652. PMID 16723392.
- 1 2 3 Métais, Grégoire (2014). On the "thumb" of anoplotheriins: a 3D comparative study of the hand of Anoplotherium and Diplobune. Swiss Geoscience Meeting 2014.
- 1 2 Abusch-Siewert, Susanne (1989). "Bemerkungen zu den Anoplotherien (Artiodactyla, Mammalia) der Pariser Gipse". Münchner Geowissenschaftlicher Abhandlungen (A). 15: 55–78.
- ↑ Heissig, Kurt (1993). "The Astragalus in Anoplotheres and Oreodonts, Phylogenetical and Paleogeographical Implications". Kaupia. 3: 173–178.
- ↑ Prothero, Donald R.; Foss, Scott E., eds. (2007). "Summary". The Evolution of Artiodactyls. Johns Hopkins University Press. p. 307.
- ↑ de Carvalho, Carlos Neto; Muñiz, Fernando; Cáceres, Luis M.; Belaústegui, Zain; Rodríguez-Vidal, Joaquín; Belo, João; Moreira, Noel; Cachão, Mário; Cunha, Pedro P.; Figueiredo, Silvério; et al. (2022). "Aurochs roamed along the SW coast of Andalusia (Spain) during Late Pleistocene". Scientific Reports. 12 (9911): 9911. Bibcode:2022NatSR..12.9911D. doi:10.1038/s41598-022-14137-6. PMC 9198092. PMID 35701579.
- 1 2 3 4 Ellenberger, Paul (1980). "Sur les empreintes de pas des gros mammifères de l'Eocène supérieur de Garrigues-ste-Eulalie (Gard)". Palaeovertebrata. 9: 37–78. Archived from the original on 2023-08-29. Retrieved 2023-08-30.
- ↑ Lucas, Spencer G.; Hunt, Adrian P. (2007). "Ichnotaxonomy of Camel Footprints". In Lucas, Spencer G.; Spielmann, Justin A.; Lockley, Martin G. (eds.). Cenozoic Vertebrate Tracks and Traces: Bulletin 42. New Mexico Museum of Natural History and Science.
- ↑ Montes, Martín Linares (2020). Paleontología e interpretación medioambiental de las icnitas de mamíferos del yacimiento de Fondota (Paleógeno, Abiego, Huesca) (PDF) (Thesis). University of Zaragoza. Archived (PDF) from the original on 2023-10-19. Retrieved 2023-08-30.
- ↑ Tsubamoto, Takehisa (2014). "Estimating body mass from the astragalus in mammals". Acta Palaeontologica Polonica. 59 (2): 259–265. doi:10.4202/app.2011.0067. S2CID 54686160.
- ↑ MacLaren, Jamie Alexander; Nauwelaerts, Sandra (2020). "Modern Tapirs as Morphofunctional Analogues for Locomotion in Endemic Eocene European Perissodactyls". Journal of Mammalian Evolution. 27 (2): 245–263. doi:10.1007/s10914-019-09460-1. hdl:10067/1580640151162165141. S2CID 254703475.
- ↑ Joomun, Sarah C.; Hooker, Jerry J.; Collinson, Margaret E. (2009). Differences in the Dietary Responses of the Perissodactyl Plagiolophus and the Artiodactyl Diplobune to the Eocene/Oligocene Transition Events in Europe (PDF). 69th Annual Meeting Society of Vertebrate Paleontology and the 57th Symposium of Vertebrate Palaeontology and Comparative Anatomy (SVPCA). Vol. 29. Archived (PDF) from the original on 2023-05-31. Retrieved 2023-08-30.
- ↑ Joomun, Sarah C.; Hooker, Jerry J.; Collinson, Margaret E. (2010). "Changes in Dental Wear of Plagiolophus Minor (Mammalia: Perissodactyla) Across the Eocene-Oligocene Transition". Journal of Vertebrate Paleontology. 30 (2): 563–576. Bibcode:2010JVPal..30..563J. doi:10.1080/02724631003618124. JSTOR 40666176. S2CID 86429890. Archived from the original on 2023-08-29. Retrieved 2023-08-30.
- ↑ Cartmill, Matt; Brown, Kaye (2017). "Posture, Locomotion and Bipedality: The Case of the Gerenuk (Litocranius walleri)". In Marom, Assaf; Hovers, Erella (eds.). Human Paleontology and Prehistory. Vertebrate Paleobiology and Paleoanthropology. Springer, Cham. pp. 53–70. doi:10.1007/978-3-319-46646-0_6. ISBN 978-3-319-46644-6.
- ↑ Clark, Ciaran; Janis, Christine M.; Hooker, Jerry J.; Rayfield, Emily J. (2018). Posture in the fossil record: can bone microstructure be used to more accurately reconstruct past behaviour? (PDF). The Palaeontological Association: 62nd Annual Meeting; 14th–17th December 2018; University of Bristol. p. 72. Archived (PDF) from the original on 2022-06-18. Retrieved 2023-08-30.
- ↑ Linares Montes, Martín; Canudo, José Ignacio; Luzón, María Aránzazu; Castanera, Diego (2021). "Un excepcional registro paleoicnológico de artiodáctilos en el Oligoceno inferior de Abiego (Huesca, España)" (PDF). Comunicações Geológicas (in Spanish). 108: 103–107. doi:10.34637/6f8b-cj24. Archived (PDF) from the original on 2023-08-29. Retrieved 2023-08-30.
- ↑ Eronen, Jussi T.; Janis, Christine M.; Chamberlain, Charles Page; Mulch, Andreas (2015). "Mountain uplift explains differences in Palaeogene patterns of mammalian evolution and extinction between North America and Europe". Proceedings of the Royal Society B. 282 (1809). doi:10.1098/rspb.2015.0136. PMC 4590438. PMID 26041349.
- 1 2 3 Maitre, Elodie (2014). "Western European middle Eocene to early Oligocene Chiroptera: systematics, phylogeny and palaeoecology based on new material from the Quercy (France)". Swiss Journal of Palaeontology. 133 (2): 141–242. Bibcode:2014SwJP..133..141M. doi:10.1007/s13358-014-0069-3. S2CID 84066785.
- 1 2 Badiola, Ainara; Perales-Gogenola, Leire; Astibia, Humberto; Suberbiola, Xabier Pereda (2022). "A synthesis of Eocene equoids (Perissodactyla, Mammalia) from the Iberian Peninsula: new signs of endemism". Historical Biology. 34 (8): 1623–1631. Bibcode:2022HBio...34.1623B. doi:10.1080/08912963.2022.2060098. S2CID 248164842.
- ↑ Robinet, Céline; Remy, Jean Albert; Laurent, Yves; Danilo, Laure; Lihoreau, Fabrice (2015). "A new genus of Lophiodontidae (Perissodactyla, Mammalia) from the early Eocene of La Borie (Southern France) and the origin of the genus Lophiodon Cuvier, 1822". Geobios. 48 (1): 25–38. Bibcode:2015Geobi..48...25R. doi:10.1016/j.geobios.2014.11.003.
- ↑ Perales-Gogenola, Leire; Badiola, Ainara; Gómez-Olivencia, Asier; Pereda-Suberbiola, Xabier (2022). "A remarkable new paleotheriid (Mammalia) in the endemic Iberian Eocene perissodactyl fauna". Journal of Vertebrate Paleontology. 42 (4). Bibcode:2022JVPal..42E9447P. doi:10.1080/02724634.2023.2189447. S2CID 258663753.
- 1 2 Solé, Floréal; Fischer, Valentin; Le Verger, Kévin; Mennecart, Bastien; Speijer, Robert P.; Peigné, Stéphane; Smith, Thierry (2022). "Evolution of European carnivorous mammal assemblages through the Paleogene". Biological Journal of the Linnean Society. 135 (4): 734–753. doi:10.1093/biolinnean/blac002.
- 1 2 Blondel, Cécile (2001). "The Eocene-Oligocene ungulates from Western Europe and their environment" (PDF). Palaeogeography, Palaeoclimatology, Palaeoecology. 168 (1–2): 125–139. Bibcode:2001PPP...168..125B. doi:10.1016/S0031-0182(00)00252-2. Archived (PDF) from the original on 2017-08-22. Retrieved 2023-08-30.
- ↑ Martin, Jeremy E.; Pochat-Cottilloux, Yohan; Laurent, Yves; Perrier, Vincent; Robert, Emmanuel; Antoine, Pierre-Olivier (2022). "Anatomy and phylogeny of an exceptionally large sebecid (Crocodylomorpha) from the middle Eocene of southern France". Journal of Vertebrate Paleontology. 42 (4). Bibcode:2022JVPal..42E3828M. doi:10.1080/02724634.2023.2193828. S2CID 258361595.
- ↑ Martin, Jeremy E. (2015). "A sebecosuchian in a middle Eocene karst with comments on the dorsal shield in Crocodylomorpha". Acta Palaeontologica Polonica. 60 (3): 673–680. doi:10.4202/app.00072.2014. S2CID 54002673.
- ↑ Antunes, Miguel Telles (2003). "Lower Paleogene Crocodilians from Silveirinha, Portugal". Palaeovenebrata. 32: 1–26. Archived from the original on 2023-08-29. Retrieved 2023-08-30.
- ↑ Bai, Bin; Wang, Yuan-Qing; Theodor, Jessica M.; Meng, Jin (2023). "Small artiodactyls with tapir-like teeth from the middle Eocene of the Erlian Basin, Inner Mongolia, China". Frontiers in Earth Science. 11: 1–20. Bibcode:2023FrEaS..1117911B. doi:10.3389/feart.2023.1117911.
- ↑ Kostopoulos, Dimitris S.; Koufos, George D.; Christanis, Kimon (2012). "On some anthracotheriid (Artiodactyla, Mammalia) remains from northern Greece: comments on the palaeozoogeography and phylogeny of Elomeryx". Swiss Journal of Palaeontology. 131 (2): 303–315. Bibcode:2012SwJP..131..303K. doi:10.1007/s13358-012-0041-z. S2CID 195363034.
- ↑ Hooker, Jerry J. (2013). "Origin and evolution of the Pseudorhyncocyonidae, a European Paleogene family of insectivorous placental mammals". Palaeontology. 56 (4): 807–835. Bibcode:2013Palgy..56..807H. doi:10.1111/pala.12018. S2CID 84322086.
- ↑ Marigó, Judit; Susanna, Ivette; Minwer-Barakat, Raef; Malapeira, Joan Madurell; Moyà-Solà, Salvador; Casanovas-Vilar, Isaac; Gimenez, Jose Maria Robles; Alba, David M. (2014). "The primate fossil record in the Iberian Peninsula". Journal of Iberian Geology. 40 (1): 179–211. doi:10.5209/rev_JIGE.2014.v40.n1.44094.
- ↑ Manz, Carly; Bloch, Jonathan Ivan (2014). "Systematics and Phylogeny of Paleocene-Eocene Nyctitheriidae (Mammalia, Eulipotyphla?) with Description of a new Species from the Late Paleocene of the Clarks Fork Basin, Wyoming, USA". Journal of Mammalian Evolution. 22 (3): 307–342. doi:10.1007/s10914-014-9284-3. S2CID 254704409.
- ↑ Badiola, Ainara; Cuesta, Miguel-Ángel (2006). "Los marsupiales del yacimiento del Eoceno Superior de Zambrana (Álava, Región Vasco-Cantábrica)". Estudios Geológicos (in Spanish). 62 (1): 349–358. doi:10.3989/egeol.0662130.
- ↑ Sigé, Bernard (1997). "Les mammiféres insectivoresdes nouvelles collections de Sossís et sites associes (Éocène supérieur, Espagne)". Geobios. 30 (1): 91–113. doi:10.1016/S0016-6995(97)80260-4.
- ↑ Dawson, Mary R. (2003). "Paleogene rodents of Eurasia". Distribution and migration of tertiary mammals in Eurasia. Vol. 10. pp. 97–127.
- ↑ Chroust, Milan; Mazuch, Martin; Luján, Àngel Hernández (2019). "New crocodilian material from the Eocene-Oligocene transition of the NW Bohemia (Czech Republic): an updated fossil record in Central Europe during the Grande Coupure". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 293 (1): 73–82. doi:10.1127/njgpa/2019/0832. S2CID 199104151.
- ↑ Rage, Jean-Claude (2012). "Amphibians and squamates in the Eocene of Europe: what do they tell us?". Palaeobiodiversity and Palaeoenvironments. 92 (4): 445–457. Bibcode:2012PdPe...92..445R. doi:10.1007/s12549-012-0087-3. S2CID 128651937.
- ↑ Minwer-Barakat, Raef; Badiola, Ainara; Marigó, Judit; Moyà-Solà, Salvador (2013). "First record of the genus Microchoerus (Omomyidae, Primates) in the western Iberian Peninsula and its palaeobiogeographic implications". Journal of Human Evolution. 65 (3): 313–321. doi:10.1016/j.jhevol.2013.07.002. PMID 23916791.
- 1 2 Aguilar, Jean-Pierre; Legendre, Serge; Michaux, Jacques (1997). "Synthèses et tableaux de corrélations". Actes du Congrès Bio-chroM'97. Mémoires et Travaux de l'EPHE Institut de Montpellier 21 (in French). École Pratique des Hautes Études-Sciences de la Vie et de la Terre, Montpellier. pp. 769–850.
- ↑ Sun, Jimin; Ni, Xijun; Bi, Shundong; Wu, Wenyu; Ye, Jie; Meng, Jin; Windley, Brian F. (2014). "Synchronous turnover of flora, fauna, and climate at the Eocene-Oligocene Boundary in Asia". Scientific Reports. 4 (7463): 7463. Bibcode:2014NatSR...4E7463S. doi:10.1038/srep07463. PMC 4264005. PMID 25501388.
- 1 2 3 4 Hooker, Jerry J.; Collinson, Margaret E.; Sille, Nicholas P. (2004). "Eocene–Oligocene mammalian faunal turnover in the Hampshire Basin, UK: calibration to the global time scale and the major cooling event" (PDF). Journal of the Geological Society. 161 (2): 161–172. Bibcode:2004JGSoc.161..161H. doi:10.1144/0016-764903-091. S2CID 140576090. Archived (PDF) from the original on 2023-08-08. Retrieved 2023-08-31.
- ↑ Legendre, Serge; Mourer-Chauviré, Cécile; Hugueney, Marguerite; Maitre, Elodie; Sigé, Bernard; Escarguel, Gilles (2006). "Dynamique de la diversité des mammifères et des oiseaux paléogènes du Massif Central (Quercy et Limagnes, France)". STRATA. 1 (in French). 13: 275–282.
- ↑ Escarguel, Gilles; Legendre, Serge; Sigé, Bernard (2008). "Unearthing deep-time biodiversity changes: The Palaeogene mammalian metacommunity of the Quercy and Limagne area (Massif Central, France)". Comptes Rendus Geoscience. 340 (9–10): 602–614. Bibcode:2008CRGeo.340..602E. doi:10.1016/j.crte.2007.11.005. Archived from the original on 2023-10-13. Retrieved 2023-09-19.
- 1 2 Costa, Elisenda; Garcés, Miguel; Sáez, Alberto; Cabrera, Lluís; López-Blanco, Miguel (2011). "The age of the "Grande Coupure" mammal turnover: New constraints from the Eocene–Oligocene record of the Eastern Ebro Basin (NE Spain)". Palaeogeography, Palaeoclimatology, Palaeoecology. 301 (1–4): 97–107. Bibcode:2011PPP...301...97C. doi:10.1016/j.palaeo.2011.01.005. hdl:2445/34510.
- ↑ Hutchinson, David K.; Coxall, Helen K.; Lunt, Daniel J.; Steinthorsdottir, Margret; De Boer, Agatha M.; Baatsen, Michiel L.J.; Von der Heydt, Anna S.; Huber, Matthew; Kennedy-Asser, Alan T.; Kunzmann, Lutz; Ladant, Jean-Baptiste; Lear, Caroline; Moraweck, Karolin; Pearson, Paul; Piga, Emanuela; Pound, Matthew J.; Salzmann, Ulrich; Scher, Howie D.; Sijp, Willem P.; Śliwińska, Kasia K; Wilson, Paul A.; Zhang, Zhongshi (2021). "The Eocene-Oligocene transition: A review of marine and terrestrial proxy data, models and model-data comparisons". Climate of the Past. 17 (1): 269–315. Bibcode:2021CliPa..17..269H. doi:10.5194/cp-17-269-2021. S2CID 234099337.
- ↑ Toumoulin, Agathe; Tardif, Delphine; Donnadieu, Yannick; Licht, Alexis; Ladant, Jean-Baptiste; Kunzmann, Lutz; Dupont-Nivet, Guillaume (2022). "Evolution of continental temperature seasonality from the Eocene greenhouse to the Oligocene icehouse –a model–data comparison". Climate of the Past. 18 (2): 341–362. Bibcode:2022CliPa..18..341T. doi:10.5194/cp-18-341-2022.
- ↑ Boulila, Slah; Dupont-Nivet, Guillaume; Galbrun, Bruno; Bauer, Hugues; Châteauneuf, Jean-Jacques (2021). "Age and driving mechanisms of the Eocene–Oligocene transition from astronomical tuning of a lacustrine record (Rennes Basin, France)". Climate of the Past. 17 (6): 2343–2360. Bibcode:2021CliPa..17.2343B. doi:10.5194/cp-17-2343-2021. S2CID 244097729.
- ↑ Rivals, Florent; Belyaev, Ruslan I.; Basova, Vera B.; Prilepskaya, Natalya E. (2023). "Hogs, hippos or bears? Paleodiet of European Oligocene anthracotheres and entelodonts". Palaeogeography, Palaeoclimatology, Palaeoecology. 611: 111363. Bibcode:2023PPP...61111363R. doi:10.1016/j.palaeo.2022.111363. S2CID 254801829.
- ↑ Becker, Damien (2009). "Earliest record of rhinocerotoids (Mammalia: Perissodactyla) from Switzerland: systematics and biostratigraphy". Swiss Journal of Geosciences. 102 (3): 489–504. doi:10.1007/s00015-009-1330-4. S2CID 67817430.
- ↑ Solé, Floréal; Fischer, Fischer; Denayer, Julien; Speijer, Robert P.; Fournier, Morgane; Le Verger, Kévin; Ladevèze, Sandrine; Folie, Annelise; Smith, Thierry (2020). "The upper Eocene-Oligocene carnivorous mammals from the Quercy Phosphorites (France) housed in Belgian collections". Geologica Belgica. 24 (1–2): 1–16. doi:10.20341/gb.2020.006. S2CID 224860287.
- ↑ Kocsis, László; Ozsvárt, Péter; Becker, Damien; Ziegler, Reinhard; Scherler, Laureline; Vlad, Codrea A. (2014). "Orogeny forced terrestrial climate variation during the late Eocene–early Oligocene in Europe" (PDF). Geology. 42 (8): 727–730. Bibcode:2014Geo....42..727K. doi:10.1130/G35673.1. Archived (PDF) from the original on 2017-09-21. Retrieved 2023-09-19.
- ↑ Hooker, Jerry J. (2010). "The 'Grande Coupure' in the Hampshire Basin, UK: taxonomy and stratigraphy of the mammals on either side of this major Palaeogene faunal turnover". In Whittaker, John E.; Hart, Malcolm B. (eds.). Micropalaeontology, Sedimentary Environments and Stratigraphy: a Tribute to Dennis Curry (1912–2001). Vol. 4. Geological Society of London. pp. 147–215. doi:10.1144/TMS004.8. ISBN 9781862396227.
- ↑ Joomun, Sarah C.; Hooker, Jerry J.; Collinson, Margaret E. (2010). Climate Change Versus Competition as the Cause of Ungulate Extinction at the Grande Coupure (Early Oligocene, Europe) (PDF). International Palaeontological Congress: London 2010. p. 221. Archived (PDF) from the original on 2020-06-11. Retrieved 2023-08-31.
External links
![]() Media related to Anoplotherium at Wikimedia Commons
Media related to Anoplotherium at Wikimedia Commons