 | |
| Names | |
|---|---|
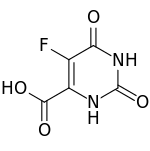
| Preferred IUPAC name
5-Fluoro-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.798 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H3FN2O4 | |
| Molar mass | 174.087 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5-Fluoroorotic acid (5FOA) is a fluorinated derivative of the pyrimidine precursor orotic acid. It is used in yeast genetics to select for the absence of the URA3 gene, which encodes the enzyme for the decarboxylation of 5-fluoroorotic acid to 5-fluorouracil, a toxic metabolite.[1] It has also been used in diatom selection.[2]
See also
References
- ↑ Boeke, J. D.; Trueheart, J.; Natsoulis, G.; Fink, G. R. (1987). "5-Fluoroorotic acid as a selective agent in yeast molecular genetics". Recombinant DNA Part E. Methods in Enzymology. Vol. 154. pp. 164–75. doi:10.1016/0076-6879(87)54076-9. ISBN 9780121820558. PMID 3323810.
- ↑ Serif, Manuel; Dubois, Gwendoline; Finoux, Anne-Laure; Teste, Marie-Ange; Jallet, Denis; Daboussi, Fayza (2018-09-25). "One-step generation of multiple gene knock-outs in the diatom Phaeodactylum tricornutum by DNA-free genome editing". Nature Communications. Springer Science and Business Media LLC. 9 (1): 3924. Bibcode:2018NatCo...9.3924S. doi:10.1038/s41467-018-06378-9. ISSN 2041-1723. PMC 6156588. PMID 30254261.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.