 | |
| Names | |
|---|---|
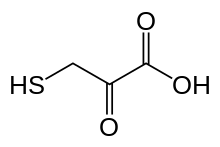
| Preferred IUPAC name
2-Oxo-3-sulfanylpropanoic acid | |
| Other names
3-Mercapto-2-oxopropanoic acid 3-MPV 3-MP | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | 3-mercaptopyruvic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4O3S | |
| Molar mass | 120.12 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3-Mercaptopyruvic acid is an intermediate in cysteine metabolism. It has been studied as a potential treatment for cyanide poisoning, but its half-life is too short for it to be clinically effective.[1] Instead, prodrugs, such as sulfanegen, are being evaluated to compensate for the short half-life of 3-mercaptopyruvic acid.[2]
See also
References
- ↑ Nagahara, N; Li, Q; Sawada, N (2003). "Do antidotes for acute cyanide poisoning act on mercaptopyruvate sulfurtransferase to facilitate detoxification?". Current Drug Targets. Immune, Endocrine and Metabolic Disorders. 3 (3): 198–204. doi:10.2174/1568008033340162. PMID 12871026.
- ↑ Brenner, M; Kim, JG; Lee, J; Mahon, SB; Lemor, D; Ahdout, R; Boss, GR; Blackledge, W; Jann, L; Nagasawa, HT; Patterson, SE (2010). "Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity". Toxicology and Applied Pharmacology. 248 (3): 269–76. doi:10.1016/j.taap.2010.08.002. PMC 3382974. PMID 20705081.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.